In Vivo Activity of Metal Complexes Containing 1,10-Phenanthroline and 3,6,9-Trioxaundecanedioate Ligands against Pseudomonas aeruginosa Infection in Galleria mellonella Larvae
- PMID: 35203432
- PMCID: PMC8869450
- DOI: 10.3390/biomedicines10020222
In Vivo Activity of Metal Complexes Containing 1,10-Phenanthroline and 3,6,9-Trioxaundecanedioate Ligands against Pseudomonas aeruginosa Infection in Galleria mellonella Larvae
Abstract
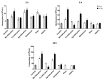
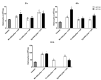
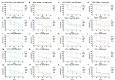
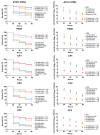
Drug-resistant Pseudomonas aeruginosa is rapidly developing resulting in a serious global threat. Immunocompromised patients are specifically at risk, especially those with cystic fibrosis (CF). Novel metal complexes incorporating 1,10-phenanthroline (phen) ligands have previously demonstrated antibacterial and anti-biofilm effects against resistant P. aeruginosa from CF patients in vitro. Herein, we present the in vivo efficacy of {[Cu(3,6,9-tdda)(phen)2]·3H2O·EtOH}n (Cu-tdda-phen), {[Mn(3,6,9-tdda)(phen)2]·3H2O·EtOH}n (Mn-tdda-phen) and [Ag2(3,6,9-tdda)(phen)4]·EtOH (Ag-tdda-phen) (tddaH2 = 3,6,9-trioxaundecanedioic acid). Individual treatments of these metal-tdda-phen complexes and in combination with the established antibiotic gentamicin were evaluated in vivo in larvae of Galleria mellonella infected with clinical isolates and laboratory strains of P. aeruginosa. G. mellonella were able to tolerate all test complexes up to 10 µg/larva. In addition, the immune response was affected by stimulation of immune cells (hemocytes) and genes that encode for immune-related peptides, specifically transferrin and inducible metallo-proteinase inhibitor. The amalgamation of metal-tdda-phen complexes and gentamicin further intensified this response at lower concentrations, clearing a P. aeruginosa infection that were previously resistant to gentamicin alone. Therefore this work highlights the anti-pseudomonal capabilities of metal-tdda-phen complexes alone and combined with gentamicin in an in vivo model.
Keywords: 1,10-phenanthroline; Galleria mellonella; Pseudomonas aeruginosa; antimicrobial resistance; in vivo model; infection; metal complexes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
The Antibacterial and Anti-biofilm Activity of Metal Complexes Incorporating 3,6,9-Trioxaundecanedioate and 1,10-Phenanthroline Ligands in Clinical Isolates of Pseudomonas Aeruginosa from Irish Cystic Fibrosis Patients.Antibiotics (Basel). 2020 Oct 5;9(10):674. doi: 10.3390/antibiotics9100674. Antibiotics (Basel). 2020. PMID: 33027987 Free PMC article.
-
Unprecedented in Vitro Antitubercular Activitiy of Manganese(II) Complexes Containing 1,10-Phenanthroline and Dicarboxylate Ligands: Increased Activity, Superior Selectivity, and Lower Toxicity in Comparison to Their Copper(II) Analogs.Front Microbiol. 2018 Jul 2;9:1432. doi: 10.3389/fmicb.2018.01432. eCollection 2018. Front Microbiol. 2018. PMID: 30013535 Free PMC article.
-
In vivo Activity of Copper(II), Manganese(II), and Silver(I) 1,10-Phenanthroline Chelates Against Candida haemulonii Using the Galleria mellonella Model.Front Microbiol. 2020 Mar 24;11:470. doi: 10.3389/fmicb.2020.00470. eCollection 2020. Front Microbiol. 2020. PMID: 32265890 Free PMC article.
-
Pseudomonas aeruginosa chromosomal beta-lactamase in patients with cystic fibrosis and chronic lung infection. Mechanism of antibiotic resistance and target of the humoral immune response.APMIS Suppl. 2003;(116):1-47. APMIS Suppl. 2003. PMID: 14692154 Review.
-
The treatment of respiratory pseudomonas infection in cystic fibrosis: what drug and which way?Drugs. 2000 Nov;60(5):1053-64. doi: 10.2165/00003495-200060050-00006. Drugs. 2000. PMID: 11129122 Review.
Cited by
-
The Lung Microbiome in COPD and Lung Cancer: Exploring the Potential of Metal-Based Drugs.Int J Mol Sci. 2023 Aug 1;24(15):12296. doi: 10.3390/ijms241512296. Int J Mol Sci. 2023. PMID: 37569672 Free PMC article. Review.
-
Label-Free Quantitative Proteomic Analysis Reveals the Effects of Biogenic Silver Nanoparticles on Fusarium keratoplasticum and Their Therapeutic Potential in Galleria mellonella Larvae.ACS Omega. 2025 Aug 14;10(33):37408-37418. doi: 10.1021/acsomega.5c03275. eCollection 2025 Aug 26. ACS Omega. 2025. PMID: 40893217 Free PMC article.
-
Silver(I) 1,10-Phenanthroline Complexes Are Active against Fonsecaea pedrosoi Viability and Negatively Modulate Its Potential Virulence Attributes.J Fungi (Basel). 2023 Mar 15;9(3):356. doi: 10.3390/jof9030356. J Fungi (Basel). 2023. PMID: 36983524 Free PMC article.
-
Clearing an ESKAPE Pathogen in a Model Organism; A Polypyridyl Ruthenium(II) Complex Theranostic that Treats a Resistant Acinetobacter baumannii Infection in Galleria mellonella.Chemistry. 2023 Feb 21;29(11):e202203555. doi: 10.1002/chem.202203555. Epub 2023 Jan 12. Chemistry. 2023. PMID: 36420820 Free PMC article.
References
-
- World Health Organization 2020 Antibacterial Agents in Clinical and Preclinical Development. [(accessed on 21 March 2021)]. Available online: https://www.who.int/publications/i/item/9789240021303.
-
- Garcia-Nuñez M., Marti S., Puig C., Perez-Brocal V., Millares L., Santos S., Ardanuy C., Moya A., Liñares J., Monsó E. Bronchial microbiome, PA biofilm-forming capacity and exacerbation in severe COPD patients colonized by P. aeruginosa. Futur. Microbiol. 2017;12:379–392. doi: 10.2217/fmb-2016-0127. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

