Antibacterial and Immunomodulatory Properties of Acellular Wharton's Jelly Matrix
- PMID: 35203437
- PMCID: PMC8869352
- DOI: 10.3390/biomedicines10020227
Antibacterial and Immunomodulatory Properties of Acellular Wharton's Jelly Matrix
Abstract
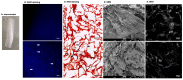
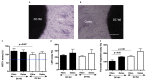
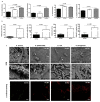
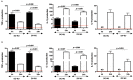
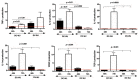
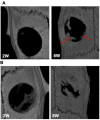
Of all biologic matrices, decellularized tissues have emerged as a promising tool in the field of regenerative medicine. Few empirical clinical studies have shown that Wharton's jelly (WJ) of the human umbilical cord promotes wound closure and reduces wound-related infections. In this scope, we herein investigated whether decellularized (DC)-WJ could be used as an engineered biomaterial. In comparison with devitalized (DV)-WJ, our results showed an inherent effect of DC-WJ on Gram positive (S. aureus and S. epidermidis) and Gram negative (E. coli and P. aeruginosa) growth and adhesion. Although DC-WJ activated the neutrophils and monocytes in a comparable magnitude to DV-WJ, macrophages modulated their phenotypes and polarization states from the resting M0 phenotype to the hybrid M1/M2 phenotype in the presence of DC-WJ. M1 phenotype was predominant in the presence of DV-WJ. Finally, the subcutaneous implantation of DC-WJ showed total resorption after three weeks of implantation without any sign of foreign body reaction. These significant data shed light on the potential regenerative application of DC-WJ in providing a suitable biomaterial for tissue regenerative medicine and an ideal strategy to prevent wound-associated infections.
Keywords: Wharton’s jelly; antibacterial; bioactivity; decellularization; immunomodulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
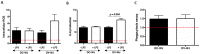

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

