Role of NLRP7 in Normal and Malignant Trophoblast Cells
- PMID: 35203462
- PMCID: PMC8868573
- DOI: 10.3390/biomedicines10020252
Role of NLRP7 in Normal and Malignant Trophoblast Cells
Abstract
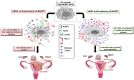
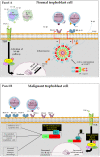
Gestational choriocarcinoma (CC) is an aggressive cancer that develops upon the occurrence of abnormal pregnancies such as Hydatidiform moles (HMs) or upon non-molar pregnancies. CC cells often metastasize in multiple organs and can cause maternal death. Recent studies have established an association between recurrent HMs and mutations in the Nlrp7 gene. NLRP7 is a member of a new family of proteins that contributes to innate immune processes. Depending on its level of expression, NLRP7 can function in an inflammasome-dependent or independent pathway. To date, the role of NLRP7 in normal and in malignant human placentation remains to be elucidated. We have recently demonstrated that NLRP7 is overexpressed in CC trophoblast cells and may contribute to their acquisition of immune tolerance via the regulation of key immune tolerance-associated factors, namely HLA family, βCG and PD-L1. We have also demonstrated that NLRP7 increases trophoblast proliferation and decreases their differentiation, both in normal and tumor conditions. Actual findings suggest that NLRP7 expression may ensure a strong tolerance of the trophoblast by the maternal immune system during normal pregnancy and may directly affect the behavior and aggressiveness of malignant trophoblast cells. The proposed review summarizes recent advances in the understanding of the significance of NLRP7 overexpression in CC and discusses its multifaceted roles, including its function in an inflammasome-dependent or independent pathways.
Keywords: NLRP7; choriocarcinoma; inflammasome; maternal immune tolerance; pregnancy; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
NLRP7 Promotes Choriocarcinoma Growth and Progression through the Establishment of an Immunosuppressive Microenvironment.Cancers (Basel). 2021 Jun 15;13(12):2999. doi: 10.3390/cancers13122999. Cancers (Basel). 2021. PMID: 34203890 Free PMC article.
-
NLRP7 Enhances Choriocarcinoma Cell Survival and Camouflage in an Inflammasome Independent Pathway.Cells. 2023 Mar 9;12(6):857. doi: 10.3390/cells12060857. Cells. 2023. PMID: 36980199 Free PMC article.
-
NLRP7 is increased in human idiopathic fetal growth restriction and plays a critical role in trophoblast differentiation.J Mol Med (Berl). 2019 Mar;97(3):355-367. doi: 10.1007/s00109-018-01737-x. Epub 2019 Jan 8. J Mol Med (Berl). 2019. PMID: 30617930
-
Genetics and Epigenetics of Recurrent Hydatidiform Moles: Basic Science and Genetic Counselling.Curr Obstet Gynecol Rep. 2014 Jan 21;3(1):55-64. doi: 10.1007/s13669-013-0076-1. eCollection 2014. Curr Obstet Gynecol Rep. 2014. PMID: 24533231 Free PMC article. Review.
-
Recurrent complete hydatidiform mole: where we are, is there a safe gestational horizon? Opinion and mini-review.J Assist Reprod Genet. 2018 Jun;35(6):967-973. doi: 10.1007/s10815-018-1202-9. Epub 2018 May 8. J Assist Reprod Genet. 2018. PMID: 29737470 Free PMC article. Review.
Cited by
-
Changes in expression levels of Nod-like receptors in the spleen of ewes.Anim Reprod. 2023 May 15;20(1):e20220093. doi: 10.1590/1984-3143-AR2022-0093. eCollection 2023. Anim Reprod. 2023. PMID: 37228386 Free PMC article.
-
NLRP7 maintains the genomic stability during early human embryogenesis via mediating alternative splicing.Commun Biol. 2025 Jan 26;8(1):125. doi: 10.1038/s42003-025-07571-5. Commun Biol. 2025. PMID: 39865169 Free PMC article.
-
Gestational Trophoblastic Disease: A Clinical Review and Case Study Presentation for the Advanced Practice Nurse.Clin J Oncol Nurs. 2025 May 19;29(3):E97-E102. doi: 10.1188/25.CJON.E97-E102. Clin J Oncol Nurs. 2025. PMID: 40401832 Review.
-
The Placental NLRP3 Inflammasome and Its Downstream Targets, Caspase-1 and Interleukin-6, Are Increased in Human Fetal Growth Restriction: Implications for Aberrant Inflammation-Induced Trophoblast Dysfunction.Cells. 2022 Apr 21;11(9):1413. doi: 10.3390/cells11091413. Cells. 2022. PMID: 35563719 Free PMC article.
-
Effects of early pregnancy on NOD-like receptor expression in the ovine endometrium.Front Vet Sci. 2024 Jun 5;11:1384386. doi: 10.3389/fvets.2024.1384386. eCollection 2024. Front Vet Sci. 2024. PMID: 38903689 Free PMC article.
References
-
- Huang X., Wang L., Zhao S., Liu H., Chen S., Wu L., Liu L., Ding J., Yang H., Maxwell A., et al. Pregnancy Induces an Immunological Memory Characterized by Maternal Immune Alterations Through Specific Genes Methylation. Front. Immunol. 2021;12:686676. doi: 10.3389/fimmu.2021.686676. - DOI - PMC - PubMed
-
- Garnier V., Traboulsi W., Salomon A., Brouillet S., Fournier T., Winkler C., Desvergne B., Hoffmann P., Zhou Q.Y., Congiu C., et al. PPARgamma controls pregnancy outcome through activation of EG-VEGF: New insights into the mechanism of placental development. Am. J. Physiol. Endocrinol. Metab. 2015;309:E357–E369. doi: 10.1152/ajpendo.00093.2015. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

