Tipping the Balance: Vitamin D Inadequacy in Children Impacts the Major Gut Bacterial Phyla
- PMID: 35203487
- PMCID: PMC8869474
- DOI: 10.3390/biomedicines10020278
Tipping the Balance: Vitamin D Inadequacy in Children Impacts the Major Gut Bacterial Phyla
Abstract
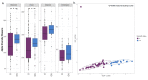
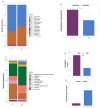
Vitamin D inadequacy appears to be on the rise globally, and it has been linked to an increased risk of osteoporosis, as well as metabolic, cardiovascular, and autoimmune diseases. Vitamin D concentrations are partially determined by genetic factors. Specific single nucleotide polymorphisms (SNPs) in genes involved in vitamin D transport, metabolism, or binding have been found to be associated with its serum concentration, and these SNPs differ among ethnicities. Vitamin D has also been suggested to be a regulator of the gut microbiota and vitamin D deficiency as the possible cause of gut microbial dysbiosis and inflammation. This pilot study aims to fill the gap in our understanding of the prevalence, cause, and implications of vitamin D inadequacy in a pediatric population residing in Qatar. Blood and fecal samples were collected from healthy subjects aged 4-14 years. Blood was used to measure serum metabolite of vitamin D, 25-hydroxycholecalciferol 25(OH)D. To evaluate the composition of the gut microbiota, fecal samples were subjected to 16S rRNA gene sequencing. High levels of vitamin D deficiency/insufficiency were observed in our cohort with 97% of the subjects falling into the inadequate category (with serum 25(OH)D < 75 nmol/L). The CT genotype in rs12512631, an SNP in the GC gene, was associated with low serum levels of vitamin D (ANOVA, p = 0.0356) and was abundant in deficient compared to non-deficient subjects. Overall gut microbial community structure was significantly different between the deficient (D) and non-deficient (ND) groups (Bray Curtis dissimilarity p = 0.049), with deficient subjects also displaying reduced gut microbial diversity. Significant differences were observed among the two major gut phyla, Firmicutes (F) and Bacteroidetes (B), where deficient subjects displayed a higher B/F ratio (p = 0.0097) compared to ND. Vitamin D deficient children also demonstrated gut enterotypes dominated by the genus Prevotella as opposed to Bacteroides. Our findings suggest that pediatric vitamin D inadequacy significantly impacts the gut microbiota. We also highlight the importance of considering host genetics and baseline gut microbiome composition in interpreting the clinical outcomes related to vitamin D deficiency as well as designing better personalized strategies for therapeutic interventions.
Keywords: Bacteroidetes to Firmicutes ratio; Qatar; gut microbiota; host genetics; pediatric vitamin D deficiency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals.Sci Rep. 2020 Dec 10;10(1):21641. doi: 10.1038/s41598-020-77806-4. Sci Rep. 2020. PMID: 33303854 Free PMC article. Clinical Trial.
-
Impact of Vitamin D Deficit on the Rat Gut Microbiome.Nutrients. 2019 Oct 24;11(11):2564. doi: 10.3390/nu11112564. Nutrients. 2019. PMID: 31652902 Free PMC article.
-
Linking serum vitamin D levels with gut microbiota after 1-year lifestyle intervention with Mediterranean diet in patients with obesity and metabolic syndrome: a nested cross-sectional and prospective study.Gut Microbes. 2023 Dec;15(2):2249150. doi: 10.1080/19490976.2023.2249150. Gut Microbes. 2023. PMID: 37647262 Free PMC article.
-
Gallstone Disease, Obesity and the Firmicutes/Bacteroidetes Ratio as a Possible Biomarker of Gut Dysbiosis.J Pers Med. 2020 Dec 25;11(1):13. doi: 10.3390/jpm11010013. J Pers Med. 2020. PMID: 33375615 Free PMC article. Review.
-
Vitamin D Deficiency in the Gulf Cooperation Council: Exploring the Triad of Genetic Predisposition, the Gut Microbiome and the Immune System.Front Immunol. 2019 May 10;10:1042. doi: 10.3389/fimmu.2019.01042. eCollection 2019. Front Immunol. 2019. PMID: 31134092 Free PMC article. Review.
Cited by
-
Immunomodulatory Role of Vitamin D on Gut Microbiome in Children.Biomedicines. 2023 May 14;11(5):1441. doi: 10.3390/biomedicines11051441. Biomedicines. 2023. PMID: 37239112 Free PMC article. Review.
-
Vitamin D, Gut Microbiota, and Cardiometabolic Diseases-A Possible Three-Way Axis.Int J Mol Sci. 2023 Jan 4;24(2):940. doi: 10.3390/ijms24020940. Int J Mol Sci. 2023. PMID: 36674452 Free PMC article. Review.
-
Gut-vitamin D interplay: key to mitigating immunosenescence and promoting healthy ageing.Immun Ageing. 2025 May 19;22(1):20. doi: 10.1186/s12979-025-00514-y. Immun Ageing. 2025. PMID: 40390005 Free PMC article. Review.
-
Probiotics and vitamins modulate the cecal microbiota of laying hens submitted to induced molting.Front Microbiol. 2023 May 9;14:1180838. doi: 10.3389/fmicb.2023.1180838. eCollection 2023. Front Microbiol. 2023. PMID: 37228378 Free PMC article.
-
Inferring diet, disease and antibiotic resistance from ancient human oral microbiomes.Microb Genom. 2024 May;10(5):001251. doi: 10.1099/mgen.0.001251. Microb Genom. 2024. PMID: 38739117 Free PMC article. Review.
References
-
- Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

