Mitochondrial Metabolism, Redox, and Calcium Homeostasis in Pulmonary Arterial Hypertension
- PMID: 35203550
- PMCID: PMC8961787
- DOI: 10.3390/biomedicines10020341
Mitochondrial Metabolism, Redox, and Calcium Homeostasis in Pulmonary Arterial Hypertension
Abstract
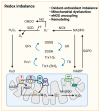
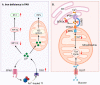
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by elevated pulmonary arterial pressure due to increased pulmonary vascular resistance, secondary to sustained pulmonary vasoconstriction and excessive obliterative pulmonary vascular remodeling. Work over the last decade has led to the identification of a critical role for metabolic reprogramming in the PAH pathogenesis. It is becoming clear that in addition to its role in ATP generation, the mitochondrion is an important organelle that regulates complex and integrative metabolic- and signal transduction pathways. This review focuses on mitochondrial metabolism alterations that occur in deranged pulmonary vessels and the right ventricle, including abnormalities in glycolysis and glucose oxidation, fatty acid oxidation, glutaminolysis, redox homeostasis, as well as iron and calcium metabolism. Further understanding of these mitochondrial metabolic mechanisms could provide viable therapeutic approaches for PAH patients.
Keywords: metabolism; mitochondria; pulmonary hypertension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tuder R.M., Archer S.L., Dorfmüller P., Erzurum S.C., Guignabert C., Michelakis E., Rabinovitch M., Schermuly R., Stenmark K.R., Morrell N.W. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J. Am. Coll. Cardiol. 2013;62((Suppl. S25)):D4–D12. doi: 10.1016/j.jacc.2013.10.025. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

