Pathophysiology of Perinatal Asphyxia in Humans and Animal Models
- PMID: 35203556
- PMCID: PMC8961792
- DOI: 10.3390/biomedicines10020347
Pathophysiology of Perinatal Asphyxia in Humans and Animal Models
Abstract
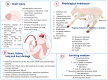
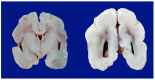
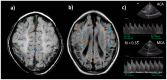
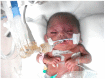
Perinatal asphyxia is caused by lack of oxygen delivery (hypoxia) to end organs due to an hypoxemic or ischemic insult occurring in temporal proximity to labor (peripartum) or delivery (intrapartum). Hypoxic-ischemic encephalopathy is the clinical manifestation of hypoxic injury to the brain and is usually graded as mild, moderate, or severe. The search for useful biomarkers to precisely predict the severity of lesions in perinatal asphyxia and hypoxic-ischemic encephalopathy (HIE) is a field of increasing interest. As pathophysiology is not fully comprehended, the gold standard for treatment remains an active area of research. Hypothermia has proven to be an effective neuroprotective strategy and has been implemented in clinical routine. Current studies are exploring various add-on therapies, including erythropoietin, xenon, topiramate, melatonin, and stem cells. This review aims to perform an updated integration of the pathophysiological processes after perinatal asphyxia in humans and animal models to allow us to answer some questions and provide an interim update on progress in this field.
Keywords: brain injury; human and animal models; hypoxic–ischemic encephalopathy; meconium aspiration syndrome; perinatal asphyxia.
Conflict of interest statement
All the authors declare that there is no conflict of interest or ethical concern in this article.
Figures




References
-
- WHO Newborns: Reducing Mortality. [(accessed on 11 November 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-morta....
-
- Bhutta Z.A., Das J.K., Bahl R., Lawn J.E., Salam R.A., Paul V.K., Sankar M.J., Blencowe H., Rizvi A., Chou V.B., et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet. 2014;384:347–370. doi: 10.1016/S0140-6736(14)60792-3. - DOI - PubMed
-
- Liu L., Johnson H.L., Cousens S., Perin J., Scott S., Lawn J.E., Rudan I., Campbell H., Cibulskis R., Li M., et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

