Decellularized Dental Pulp, Extracellular Vesicles, and 5-Azacytidine: A New Tool for Endodontic Regeneration
- PMID: 35203612
- PMCID: PMC8962372
- DOI: 10.3390/biomedicines10020403
Decellularized Dental Pulp, Extracellular Vesicles, and 5-Azacytidine: A New Tool for Endodontic Regeneration
Abstract
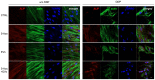
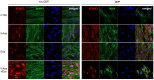
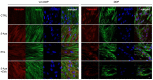
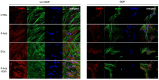
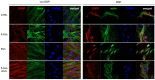
Dental pulp is a major component of the dental body that serves to maintain the tooth life and function. The aim of the present work was to develop a system that functions as a growth-permissive microenvironment for dental pulp regeneration using a decellularized dental pulp (DDP) matrix, 5-Aza-2'-deoxycytidine (5-Aza), and Extracellular Vesicles (EVs) derived from human Dental Pulp Stem Cells (hDPSCs). Human dental pulps extracted from healthy teeth, scheduled to be removed for orthodontic purpose, were decellularized and then recellularized with hDPSCs. The hDPSCs were seeded on DDP and maintained under different culture conditions: basal medium (CTRL), EVs, 5-Aza, and EVs+-5-Aza. Immunofluorescence staining and Western blot analyses were performed to evaluate the proteins' expression related to dentinogenesis, such as ALP, RUNX2, COL1A1, Vinculin, DMP1, and DSPP. Protein contents found in the DDP recellularized with hDPSCs were highly expressed in samples co-treated with EVs and 5-Aza compared to other culture conditions. This study developed a DDP matrix loaded by hDPSCs in co-treatment with EVs, which might enhance the dentinogenic differentiation with a high potentiality for endodontic regeneration.
Keywords: 5-azacytidine; decellularized dental pulp; endodontic regeneration; extracellular matrix scaffold; extracellular vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ghasemi-Mobarakeh L., Beigi M.H., Ebrahimi-Kahrizsangi R., Karbalaie K., Nasr-Esfahani M.H. Dental Pulp Stem Cells and Nanofibrous Scaffolds for Application in Tissue Engineering. Int. J. Artif. Organs. 2011;34:686.
LinkOut - more resources
Full Text Sources
Miscellaneous

