Synthesis, Structure, and Antiproliferative Action of 2-Pyridyl Urea-Based Cu(II) Complexes
- PMID: 35203671
- PMCID: PMC8962293
- DOI: 10.3390/biomedicines10020461
Synthesis, Structure, and Antiproliferative Action of 2-Pyridyl Urea-Based Cu(II) Complexes
Abstract
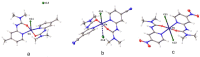
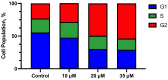
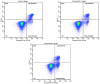
Relying on a recently suggested protocol that furnishes convenient access to variously substituted 2-pyridyl ureas, twelve hitherto unknown Cu(II) complexes have been synthesized in the present work and their structures were evaluated by elemental analysis, HRMS, IR spectroscopy, and X-ray diffraction study. Two structural motifs ([Cu(L)2Cl]+[Cl]- or (Cu(L)2Cl2) depending on the substitution pattern on the 2-pyridine fragment were revealed. In addition, antiproliferative action of the obtained compounds have been investigated against lung cancer cell lines (A549, NCI-H460, NCI-H1975), and healthy WI-26 VA4 cells were used to monitor non-specific cytotoxicity. Two nitro-group substituted complexes Cu(U3)2Cl2 (IC50 = 39.6 ± 4.5 μM) and Cu(U11)2Cl2 (IC50 = 33.4 ± 3.8 μM) demonstrate enhanced activity against the drug resistant NCI-H1975 cells with moderate selectivity toward normal WI-26 VA4 cells. The antiproliferative mechanism of cell death underlying the growth inhibitory effect of the synthesized complexes was studied via additional experiments, including the cell cycle analysis and the apoptosis induction test. Reassuringly, certain 2-pyridyl urea-based Cu(II) complexes exerted cell line-specific antiproliferative effect which renders them valuable starting points for further unveiling the anticancer potential of this class of coordination compounds.
Keywords: anti-cancer drugs; cytotoxicity; lung cancer; metal complexes; ureas.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cavalcanti I.D.L., Soares J.C.S. Advances in Cancer Treatment. Springer International Publishing; Cham, Switzerland: 2021. Conventional Chemotherapy Versus Targeted Therapy; pp. 79–89.
Grants and funding
LinkOut - more resources
Full Text Sources

