IGF-1 as a Potential Therapy for Spinocerebellar Ataxia Type 3
- PMID: 35203722
- PMCID: PMC8962315
- DOI: 10.3390/biomedicines10020505
IGF-1 as a Potential Therapy for Spinocerebellar Ataxia Type 3
Abstract
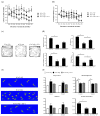
Although the effects of growth hormone (GH) therapy on spinocerebellar ataxia type 3 (SCA3) have been examined in transgenic SCA3 mice, it still poses a nonnegligible risk of cancer when used for a long term. This study investigated the efficacy of IGF-1, a downstream mediator of GH, in vivo for SCA3 treatment. IGF-1 (50 mg/kg) or saline, once a week, was intraperitoneally injected to SCA3 84Q transgenic mice harboring a human ATXN3 gene with a pathogenic expanded 84 cytosine-adenine-guanine (CAG) repeat motif at 9 months of age. Compared with the control mice harboring a 15 CAG repeat motif, the SCA3 84Q mice treated with IGF-1 for 9 months exhibited the improvement only in locomotor function and minimized degeneration of the cerebellar cortex as indicated by the survival of more Purkinje cells with a more favorable mitochondrial function along with a decrease in oxidative stress caused by DNA damage. These findings could be attributable to the inhibition of mitochondrial fission, resulting in mitochondrial fusion, and decreased immunofluorescence staining in aggresome formation and ataxin-3 mutant protein levels, possibly through the enhancement of autophagy. The findings of this study show the therapeutic potential effect of IGF-1 injection for SCA3 to prevent the exacerbation of disease progress.
Keywords: Purkinje cells; autophagy; insulin-like growth factor-1; locomotor function; mitochondrial function; spinocerebellar ataxia type 3.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






Similar articles
-
Growth hormone rescue cerebellar degeneration in SCA3 transgenic mice.Biochem Biophys Res Commun. 2020 Aug 20;529(2):467-473. doi: 10.1016/j.bbrc.2020.05.116. Epub 2020 Jul 2. Biochem Biophys Res Commun. 2020. PMID: 32703453
-
Far-infrared Radiation Improves Motor Dysfunction and Neuropathology in Spinocerebellar Ataxia Type 3 Mice.Cerebellum. 2019 Feb;18(1):22-32. doi: 10.1007/s12311-018-0936-3. Cerebellum. 2019. PMID: 29725949
-
Transcriptomic and Metabolic Network Analysis of Metabolic Reprogramming and IGF-1 Modulation in SCA3 Transgenic Mice.Int J Mol Sci. 2021 Jul 26;22(15):7974. doi: 10.3390/ijms22157974. Int J Mol Sci. 2021. PMID: 34360740 Free PMC article.
-
Progress in pathogenesis studies of spinocerebellar ataxia type 1.Philos Trans R Soc Lond B Biol Sci. 1999 Jun 29;354(1386):1079-81. doi: 10.1098/rstb.1999.0462. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10434309 Free PMC article. Review.
-
Spinocerebellar Ataxia Type 3: A Case Report and Literature Review.J Neuropathol Exp Neurol. 2020 Jun 1;79(6):641-646. doi: 10.1093/jnen/nlaa033. J Neuropathol Exp Neurol. 2020. PMID: 32346735 Review.
Cited by
-
The natural breakthrough: phytochemicals as potent therapeutic agents against spinocerebellar ataxia type 3.Sci Rep. 2024 Jan 17;14(1):1529. doi: 10.1038/s41598-024-51954-3. Sci Rep. 2024. PMID: 38233440 Free PMC article.
-
CatWalk XT gait parameters: a review of reported parameters in pre-clinical studies of multiple central nervous system and peripheral nervous system disease models.Front Behav Neurosci. 2023 Jun 7;17:1147784. doi: 10.3389/fnbeh.2023.1147784. eCollection 2023. Front Behav Neurosci. 2023. PMID: 37351154 Free PMC article. Review.
-
Neuroprotection by Drugs, Nutraceuticals and Physical Activity.Int J Mol Sci. 2023 Feb 6;24(4):3176. doi: 10.3390/ijms24043176. Int J Mol Sci. 2023. PMID: 36834601 Free PMC article.
-
The neurobiology of insulin-like growth factor I: From neuroprotection to modulation of brain states.Mol Psychiatry. 2023 Aug;28(8):3220-3230. doi: 10.1038/s41380-023-02136-6. Epub 2023 Jun 23. Mol Psychiatry. 2023. PMID: 37353586 Review.
-
A pilot study: handgrip as a predictor in the disease progression of SCA3.Orphanet J Rare Dis. 2023 Oct 11;18(1):317. doi: 10.1186/s13023-023-02948-3. Orphanet J Rare Dis. 2023. PMID: 37817286 Free PMC article.
References
-
- Wu Y., Lin H., Chen C., Gwinn K., Ro L., Wang Y., Li S., Hwang J., Fang K., Hsieh-Li H., et al. Genetic testing in spinocerebellar ataxia in Taiwan: Expansions of trinucleotide repeats in SCA8 and SCA17 are associated with typical Parkinson’s disease. Clin. Genet. 2004;65:209–214. doi: 10.1111/j.0009-9163.2004.00213.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

