Lipoxin A4 Receptor Stimulation Attenuates Neuroinflammation in a Mouse Model of Intracerebral Hemorrhage
- PMID: 35203926
- PMCID: PMC8869920
- DOI: 10.3390/brainsci12020162
Lipoxin A4 Receptor Stimulation Attenuates Neuroinflammation in a Mouse Model of Intracerebral Hemorrhage
Abstract
Intracerebral hemorrhage (ICH) is caused by the rupture of blood vessels in the brain. The excessive activation of glial cells and the infiltration of numerous inflammatory cells are observed during bleeding. Thrombin is a key molecule that triggers neuroinflammation in the ICH brain. In this study, we focused on lipoxin A4 (LXA4), an arachidonic acid metabolite that has been reported to suppress inflammation and cell migration. LXA4 and BML-111, an agonist of the LXA4 receptor/formyl peptide receptor 2 (ALX/FPR2), suppressed microglial activation; LXA4 strongly inhibited the migration of neutrophil-like cells in vitro. ALX/FPR2 was expressed on neutrophils in the ICH mouse brain and the daily administration of BML-111 attenuated the motor coordination dysfunction and suppressed the production of proinflammatory cytokines in the ICH mouse brain. On the other hand, BML-111 did not show a significant reduction in the number of microglia and neutrophils. These results suggest that systemic administration of ALX/FPR2 agonists may suppress the neuroinflammatory response of microglia and neutrophils without a change in cell numbers. Additionally, their combination with molecules that reduce cell numbers, such as modulators of leukotriene B4 signaling, may be required in future studies.
Keywords: intracerebral hemorrhage; lipoxin A4; microglia; neuroinflammation; neutrophil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
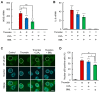
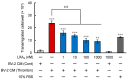

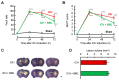
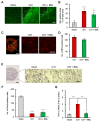
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

