Nonequivalent After-Effects of Alternating Current Stimulation on Motor Cortex Oscillation and Inhibition: Simulation and Experimental Study
- PMID: 35203958
- PMCID: PMC8870173
- DOI: 10.3390/brainsci12020195
Nonequivalent After-Effects of Alternating Current Stimulation on Motor Cortex Oscillation and Inhibition: Simulation and Experimental Study
Abstract
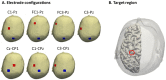
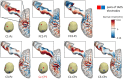
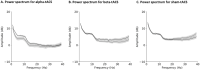
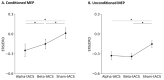
The effects of transcranial alternating current stimulation (tACS) frequency on brain oscillations and cortical excitability are still controversial. Therefore, this study investigated how different tACS frequencies differentially modulate cortical oscillation and inhibition. To do so, we first determined the optimal positioning of tACS electrodes through an electric field simulation constructed from magnetic resonance images. Seven electrode configurations were tested on the electric field of the precentral gyrus (hand motor area). We determined that the Cz-CP1 configuration was optimal, as it resulted in higher electric field values and minimized the intra-individual differences in the electric field. Therefore, tACS was delivered to the hand motor area through this arrangement at a fixed frequency of 10 Hz (alpha-tACS) or 20 Hz (beta-tACS) with a peak-to-peak amplitude of 0.6 mA for 20 min. We found that alpha- and beta-tACS resulted in larger alpha and beta oscillations, respectively, compared with the oscillations observed after sham-tACS. In addition, alpha- and beta-tACS decreased the amplitudes of conditioned motor evoked potentials and increased alpha and beta activity, respectively. Correspondingly, alpha- and beta-tACSs enhanced cortical inhibition. These results show that tACS frequency differentially affects motor cortex oscillation and inhibition.
Keywords: electric field simulation; oscillation; primary motor cortex; spike-timing-dependent plasticity; transcranial alternating current stimulation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

