Calycosin Alleviates Paraquat-Induced Neurodegeneration by Improving Mitochondrial Functions and Regulating Autophagy in a Drosophila Model of Parkinson's Disease
- PMID: 35204105
- PMCID: PMC8868496
- DOI: 10.3390/antiox11020222
Calycosin Alleviates Paraquat-Induced Neurodegeneration by Improving Mitochondrial Functions and Regulating Autophagy in a Drosophila Model of Parkinson's Disease
Abstract
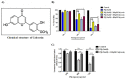
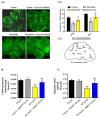
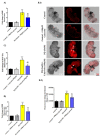
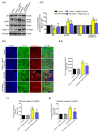
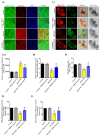
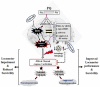
Parkinson's disease (PD) is the second most common age-related neurodegenerative disorder with limited clinical treatments. The occurrence of PD includes both genetic and environmental toxins, such as the pesticides paraquat (PQ), as major contributors to PD pathology in both invertebrate and mammalian models. Calycosin, an isoflavone phytoestrogen, has multiple pharmacological properties, including neuroprotective activity. However, the paucity of information regarding the neuroprotective potential of calycosin on PQ-induced neurodegeneration led us to explore whether calycosin can mitigate PD-like phenotypes and the underlying molecular mechanisms. We used a PQ-induced PD model in Drosophila as a cost-effective in vivo screening platform to investigate the neuroprotective efficacy of natural compounds on PD. We reported that calycosin shows a protective role in preventing dopaminergic (DA) neuronal cell death in PQ-exposed Canton S flies. Calycosin-fed PQ-exposed flies exhibit significant resistance against PQ-induced mortality and locomotor deficits in terms of reduced oxidative stress, loss of DA neurons, the depletion of dopamine content, and phosphorylated JNK-caspase-3 levels. Additionally, mechanistic studies show that calycosin administration improves PQ-induced mitochondrial dysfunction and stimulates mitophagy and general autophagy with reduced pS6K and p4EBP1 levels, suggestive of a maintained energy balance between anabolic and catabolic processes, resulting in the inhibition of neuronal cell death. Collectively, this study substantiates the protective effect of calycosin against PQ-induced neurodegeneration by improving DA neurons' survival and reducing apoptosis, likely via autophagy induction, and it is implicated as a novel therapeutic application against toxin-induced PD pathogenesis.
Keywords: Drosophila; Parkinson’s disease; autophagy; calycosin; neurodegeneration; paraquat.
Conflict of interest statement
All authors have declared no conflict of interest.
Figures



Similar articles
-
Mutation in Drosophila methuselah resists paraquat induced Parkinson-like phenotypes.Neurobiol Aging. 2014 Oct;35(10):2419.e1-2419.e16. doi: 10.1016/j.neurobiolaging.2014.04.008. Epub 2014 Apr 18. Neurobiol Aging. 2014. PMID: 24819147
-
Naringenin alleviates paraquat-induced dopaminergic neuronal loss in SH-SY5Y cells and a rat model of Parkinson's disease.Neuropharmacology. 2021 Dec 15;201:108831. doi: 10.1016/j.neuropharm.2021.108831. Epub 2021 Oct 13. Neuropharmacology. 2021. PMID: 34655599
-
Neuroprotective action of 4-Hydroxyisophthalic acid against paraquat-induced motor impairment involves amelioration of mitochondrial damage and neurodegeneration in Drosophila.Neurotoxicology. 2018 May;66:160-169. doi: 10.1016/j.neuro.2018.04.006. Epub 2018 Apr 10. Neurotoxicology. 2018. PMID: 29653138
-
Revisiting the Paraquat-Induced Sporadic Parkinson's Disease-Like Model.Mol Neurobiol. 2019 Feb;56(2):1044-1055. doi: 10.1007/s12035-018-1148-z. Epub 2018 Jun 3. Mol Neurobiol. 2019. PMID: 29862459 Review.
-
Therapeutic potentials of plant iridoids in Alzheimer's and Parkinson's diseases: A review.Eur J Med Chem. 2019 May 1;169:185-199. doi: 10.1016/j.ejmech.2019.03.009. Epub 2019 Mar 8. Eur J Med Chem. 2019. PMID: 30877973 Review.
Cited by
-
Cytotoxicity of Callerya speciosa Fractions against Myeloma and Lymphoma Cell Lines.Molecules. 2022 Apr 3;27(7):2322. doi: 10.3390/molecules27072322. Molecules. 2022. PMID: 35408721 Free PMC article.
-
Autophagy and neuroprotection in astrocytes exposed to 6-hydroxydopamine is negatively regulated by NQO2: relevance to Parkinson's disease.Sci Rep. 2023 Dec 7;13(1):21624. doi: 10.1038/s41598-023-44666-7. Sci Rep. 2023. PMID: 38062122 Free PMC article.
-
Fluorescence microscopy-based sensitive method to quantify dopaminergic neurodegeneration in a Drosophila model of Parkinson's disease.Front Neurosci. 2023 Jun 26;17:1158858. doi: 10.3389/fnins.2023.1158858. eCollection 2023. Front Neurosci. 2023. PMID: 37434762 Free PMC article.
-
Neuropharmacological effects of calycosin: a translational review of molecular mechanisms and therapeutic applications.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr 16. doi: 10.1007/s00210-025-04154-3. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40237798 Review.
-
DJ-1 and SOD1 Act Independently in the Protection against Anoxia in Drosophila melanogaster.Antioxidants (Basel). 2022 Aug 5;11(8):1527. doi: 10.3390/antiox11081527. Antioxidants (Basel). 2022. PMID: 36009245 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

