Cellular Localization of Kynurenine 3-Monooxygenase in the Brain: Challenging the Dogma
- PMID: 35204197
- PMCID: PMC8868204
- DOI: 10.3390/antiox11020315
Cellular Localization of Kynurenine 3-Monooxygenase in the Brain: Challenging the Dogma
Abstract
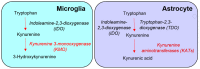
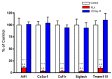
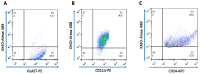
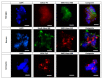
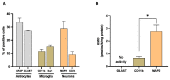
Kynurenine 3-monooxygenase (KMO), a key player in the kynurenine pathway (KP) of tryptophan degradation, regulates the synthesis of the neuroactive metabolites 3-hydroxykynurenine (3-HK) and kynurenic acid (KYNA). KMO activity has been implicated in several major brain diseases including Huntington's disease (HD) and schizophrenia. In the brain, KMO is widely believed to be predominantly localized in microglial cells, but verification in vivo has not been provided so far. Here, we examined KP metabolism in the brain after depleting microglial cells pharmacologically with the colony stimulating factor 1 receptor inhibitor PLX5622. Young adult mice were fed PLX5622 for 21 days and were euthanized either on the next day or after receiving normal chow for an additional 21 days. Expression of microglial marker genes was dramatically reduced on day 22 but had fully recovered by day 43. In both groups, PLX5622 treatment failed to affect Kmo expression, KMO activity or tissue levels of 3-HK and KYNA in the brain. In a parallel experiment, PLX5622 treatment also did not reduce KMO activity, 3-HK and KYNA in the brain of R6/2 mice (a model of HD with activated microglia). Finally, using freshly isolated mouse cells ex vivo, we found KMO only in microglia and neurons but not in astrocytes. Taken together, these data unexpectedly revealed that neurons contain a large proportion of functional KMO in the adult mouse brain under both physiological and pathological conditions.
Keywords: Huntington’s disease; astrocyte; kynurenine pathway; microglia; schizophrenia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hirai K., Kuroyanagi H., Tatebayashi Y., Hayashi Y., Hirabayashi-Takahashi K., Saito K., Haga S., Uemura T., Izumi S. Dual role of the carboxyl-terminal region of pig liver L-kynurenine 3-monooxygenase: Mitochondrial-targeting signal and enzymatic activity. J. Biochem. 2010;148:639–650. doi: 10.1093/jb/mvq099. - DOI - PubMed
-
- Quan G.X., Kim I., Komoto N., Sezutsu H., Ote M., Shimada T., Kanda T., Mita K., Kobayashi M., Tamura T. Characterization of the kynurenine 3-monooxygenase gene corresponding to the white egg 1 mutant in the silkworm Bombyx mori. Mol. Genet. Genom. 2002;267:1–9. doi: 10.1007/s00438-001-0629-2. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

