Antioxidant Activity of Natural Hydroquinones
- PMID: 35204225
- PMCID: PMC8868229
- DOI: 10.3390/antiox11020343
Antioxidant Activity of Natural Hydroquinones
Abstract
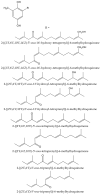
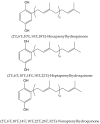
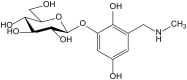
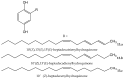
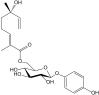
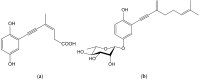
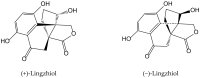
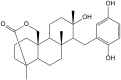
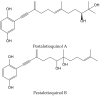
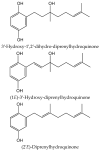
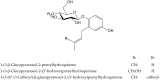
Secondary metabolites derived from hydroquinone are quite rare in nature despite the original simplicity of its structure, especially when compared to other derivatives with which it shares biosynthetic pathways. However, its presence in a prenylated form is somewhat relevant, especially in the marine environment, where it is found in different algae and invertebrates. Sometimes, more complex molecules have also been identified, as in the case of polycyclic diterpenes, such as those possessing an abietane skeleton. In every case, the presence of the dihydroxy group in the para position gives them antioxidant capacity, through its transformation into para-quinones.This review focuses on natural hydroquinones with antioxidant properties referenced in the last fifteen years. This activity, which has been generally demonstrated in vitro, should lead to relevant pharmacological properties, through its interaction with enzymes, transcription factors and other proteins, which may be particularly relevant for the prevention of degenerative diseases of the central nervous system, or also in cancer and metabolic or immune diseases. As a conclusion, this review has updated the pharmacological potential of hydroquinone derivatives, despite the fact that only a small number of molecules are known as active principles in established medicinal plants. The highlights of the present review are as follows: (a) sesquiterpenoid zonarol and analogs, whose activity is based on the stimulation of the Nrf2/ARE pathway, have a neuroprotective effect; (b) the research on pestalotioquinol and analogs (aromatic ene-ynes) in the pharmacology of atherosclerosis is of great value, due to their agonistic interaction with LXRα; and (c) prenylhydroquinones with a selective effect on tyrosine nitration or protein carbonylation may be of interest in the control of post-translational protein modifications, which usually appear in chronic inflammatory diseases.
Keywords: alkyl phenolics; antioxidant; hydroquinones; marine natural products; meroterpenoids; reactive oxygen species; secondary metabolites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

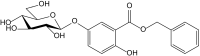
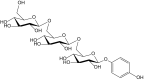

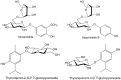
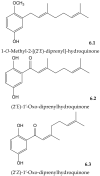





References
-
- Ohsugi M., Fan W., Hase K., Xiong Q., Tezuka Y., Komatsu K., Namba T., Saitoh T., Tazawa K., Kadot S. Active-oxygen scavenging activity of traditional nourishing-tonic herbal medicines and active constituents of Rhodiola sacra. J. Ethnopharmacol. 1999;67:111–119. doi: 10.1016/S0378-8741(98)00245-1. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

