HNO Protects the Myocardium against Reperfusion Injury, Inhibiting the mPTP Opening via PKCε Activation
- PMID: 35204265
- PMCID: PMC8869498
- DOI: 10.3390/antiox11020382
HNO Protects the Myocardium against Reperfusion Injury, Inhibiting the mPTP Opening via PKCε Activation
Abstract
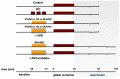
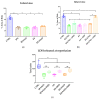
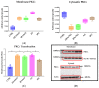
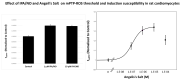
Donors of nitroxyl (HNO), the one electron-reduction product of nitric oxide (NO.), positively modulate cardiac contractility/relaxation while limiting ischemia-reperfusion (I/R) injury. The mechanisms underpinning HNO anti-ischemic effects remain poorly understood. Using isolated perfused rat hearts subjected to 30 min global ischemia/1 or 2 h reperfusion, here we tested whether, in analogy to NO., HNO protection requires PKCε translocation to mitochondria and KATP channels activation. To this end, we compared the benefits afforded by ischemic preconditioning (IPC; 3 cycles of I/R) with those eventually granted by the NO. donor, diethylamine/NO, DEA/NO, and two chemically unrelated HNO donors: Angeli's salt (AS, a prototypic donor) and isopropylamine/NO (IPA/NO, a new HNO releaser). All donors were given for 19 min before I/R injury. In control I/R hearts (1 h reperfusion), infarct size (IS) measured via tetrazolium salt staining was 66 ± 5.5% of the area at risk. Both AS and IPA/NO were as effective as IPC in reducing IS [30.7 ± 2.2 (AS), 31 ± 2.9 (IPA/NO), and 31 ± 0.8 (IPC), respectively)], whereas DEA/NO was significantly less so (36.2 ± 2.6%, p < 0.001 vs. AS, IPA/NO, or IPC). IPA/NO protection was still present after 120 min of reperfusion, and the co-infusion with the PKCε inhibitor (PKCV1-2500 nM) prevented it (IS = 30 ± 0.5 vs. 61 ± 1.8% with IPA/NO alone, p < 0.01). Irrespective of the donor, HNO anti-ischemic effects were insensitive to the KATP channel inhibitor, 5-OH decanoate (5HD, 100 μM), that, in contrast, abrogated DEA/NO protection. Finally, both HNO donors markedly enhanced the mitochondrial permeability transition pore (mPTP) ROS threshold over control levels (≅35-40%), an action again insensitive to 5HD. Our study shows that HNO donors inhibit mPTP opening, thus limiting myocyte loss at reperfusion, a beneficial effect that requires PKCε translocation to the mitochondria but not mitochondrial K+ channels activation.
Keywords: KATP channels; PKCε; mitochondrial permeability transition pore (mPTP); myocardial reperfusion injury; nitric oxide (NO.); nitroxyl (HNO).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning.Free Radic Biol Med. 2003 Jan 1;34(1):33-43. doi: 10.1016/s0891-5849(02)01179-6. Free Radic Biol Med. 2003. PMID: 12498977
-
Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning?Cardiovasc Res. 2002 Aug 15;55(3):534-43. doi: 10.1016/s0008-6363(02)00455-8. Cardiovasc Res. 2002. PMID: 12160950
-
Morphine Postconditioning Protects Against Reperfusion Injury: the Role of Protein Kinase C-Epsilon, Extracellular Signal-Regulated Kinase 1/2 and Mitochondrial Permeability Transition Pores.Cell Physiol Biochem. 2016;39(5):1930-1940. doi: 10.1159/000447890. Epub 2016 Oct 24. Cell Physiol Biochem. 2016. PMID: 27771708
-
The emergence of nitroxyl (HNO) as a pharmacological agent.Biochim Biophys Acta. 2009 Jul;1787(7):835-40. doi: 10.1016/j.bbabio.2009.04.015. Epub 2009 May 6. Biochim Biophys Acta. 2009. PMID: 19426703 Free PMC article. Review.
-
Gene expression profiles of NO- and HNO-donor treated breast cancer cells: insights into tumor response and resistance pathways.Nitric Oxide. 2014 Dec 1;43:17-28. doi: 10.1016/j.niox.2014.08.003. Epub 2014 Aug 19. Nitric Oxide. 2014. PMID: 25153034 Free PMC article. Review.
Cited by
-
Gasotransmitters and noble gases in cardioprotection: unraveling molecular pathways for future therapeutic strategies.Basic Res Cardiol. 2024 Aug;119(4):509-544. doi: 10.1007/s00395-024-01061-1. Epub 2024 Jun 15. Basic Res Cardiol. 2024. PMID: 38878210 Free PMC article. Review.
-
Platelets and Cardioprotection: The Role of Nitric Oxide and Carbon Oxide.Int J Mol Sci. 2023 Mar 24;24(7):6107. doi: 10.3390/ijms24076107. Int J Mol Sci. 2023. PMID: 37047079 Free PMC article. Review.
-
Clinical Applications for Gasotransmitters in the Cardiovascular System: Are We There Yet?Int J Mol Sci. 2023 Aug 5;24(15):12480. doi: 10.3390/ijms241512480. Int J Mol Sci. 2023. PMID: 37569855 Free PMC article. Review.
-
Sulfide regulation and catabolism in health and disease.Signal Transduct Target Ther. 2025 May 30;10(1):174. doi: 10.1038/s41392-025-02231-w. Signal Transduct Target Ther. 2025. PMID: 40442106 Free PMC article. Review.
-
Curcumin pretreatment attenuates myocardial ischemia/reperfusion injury by inhibiting ferroptosis, autophagy and apoptosis via HES1.Int J Mol Med. 2024 Dec;54(6):110. doi: 10.3892/ijmm.2024.5434. Epub 2024 Oct 4. Int J Mol Med. 2024. PMID: 39364745 Free PMC article.
References
-
- Paolocci N., Biondi R., Bettini M., Lee C.I., Berlowitz C.O., Rossi R., Xia Y., Ambrosio G., L’Abbate A., Kass D.A., et al. Oxygen radical-mediated reduction in basal and agonist-evoked no release in isolated rat heart. J. Mol. Cell. Cardiol. 2001;33:671–679. doi: 10.1006/jmcc.2000.1334. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

