The Role of Selenoprotein Tissue Homeostasis in MetS Programming: Energy Balance and Cardiometabolic Implications
- PMID: 35204276
- PMCID: PMC8869711
- DOI: 10.3390/antiox11020394
The Role of Selenoprotein Tissue Homeostasis in MetS Programming: Energy Balance and Cardiometabolic Implications
Abstract
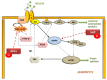
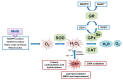
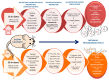
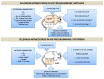
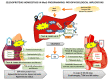
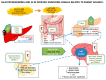
Selenium (Se) is an essential trace element mainly known for its antioxidant, anti-inflammatory, and anti-apoptotic properties, as it is part of the catalytic center of 25 different selenoproteins. Some of them are related to insulin resistance (IR) and metabolic syndrome (MetS) generation, modulating reactive oxygen species (ROS), and the energetic sensor AMP-activated protein kinase (AMPK); they can also regulate the nuclear transcription factor kappa-B (NF-kB), leading to changes in inflammation production. Selenoproteins are also necessary for the correct synthesis of insulin and thyroid hormones. They are also involved in endocrine central regulation of appetite and energy homeostasis, affecting growth and development. MetS, a complex metabolic disorder, can appear during gestation and lactation in mothers, leading to energetic and metabolic changes in their offspring that, according to the metabolic programming theory, will produce cardiovascular and metabolic diseases later in life. However, there is a gap concerning Se tissue levels and selenoproteins' implications in MetS generation, which is even greater during MetS programming. This narrative review also provides an overview of the existing evidence, based on experimental research from our laboratory, which strengthens the fact that maternal MetS leads to changes in Se tissue deposits and antioxidant selenoproteins' expression in their offspring. These changes contribute to alterations in tissues' oxidative damage, inflammation, energy balance, and tissue function, mainly in the heart. Se imbalance also could modulate appetite and endocrine energy balance, affecting pups' growth and development. MetS pups present a profile similar to that of diabetes type 1, which also appeared when dams were exposed to low-Se dietary supply. Maternal Se supplementation should be taken into account if, during gestation and/or lactation periods, there are suspicions of endocrine energy imbalance in the offspring, such as MetS. It could be an interesting therapy to induce heart reprogramming. However, more studies are necessary.
Keywords: cardiovascular disease; fetal programming; metabolic syndrome; selenium; selenoprotein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

