Culture Conditions Affect Antioxidant Production, Metabolism and Related Biomarkers of the Microalgae Phaeodactylum tricornutum
- PMID: 35204292
- PMCID: PMC8869413
- DOI: 10.3390/antiox11020411
Culture Conditions Affect Antioxidant Production, Metabolism and Related Biomarkers of the Microalgae Phaeodactylum tricornutum
Abstract
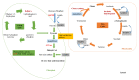
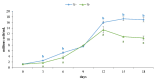
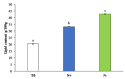
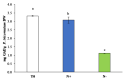
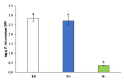
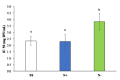
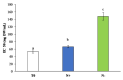
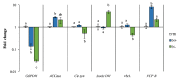
Phaeodactylum tricornutum (Bacillariophyta) is a worldwide-distributed diatom with the ability to adapt and survive in different environmental habitats and nutrient-limited conditions. In this research, we investigated the growth performance, the total lipids productivity, the major categories of fatty acids, and the antioxidant content in P. tricornutum subjected for 15 days to nitrogen deprivation (N-) compared to standard culture conditions (N+). Furthermore, genes and pathways related to lipid biosynthesis (i.e., glucose-6-phosphate dehydrogenase, acetyl-coenzyme A carboxylase, citrate synthase, and isocitrate dehydrogenase) and photosynthetic activity (i.e., ribulose-1,5-bisphospate carboxylase/oxygenase and fucoxanthin-chlorophyll a/c binding protein B) were investigated through molecular approaches. P. tricornutum grown under starvation condition (N-) increased lipids production (42.5 ± 0.19 g/100 g) and decreased secondary metabolites productivity (phenolic content: 3.071 ± 0.17 mg GAE g-1; carotenoids: 0.35 ± 0.01 mg g-1) when compared to standard culture conditions (N+). Moreover, N deprivation led to an increase in the expression of genes involved in fatty acid biosynthesis and a decrease in genes related to photosynthesis. These results could be used as indicators of nitrogen limitation for environmental or industrial monitoring of P. tricornutum.
Keywords: Phaeodactylum tricornutum; antioxidant activity; gene expression; lipid biosynthesis; nitrogen stress; photosynthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Andrade D.S., Amaral H.F., Gavilanes F.Z., Morioka L.R.I., Nassar J.M., de Melo J.M., Silva H.R., Telles T.S. Advances in the Domain of Environmental Biotechnology. Springer; Singapore: 2021. Microalgae: Cultivation, Biotechnological, Environmental, and Agricultural Applications; pp. 635–701.
-
- Maltsev Y., Maltseva K. Fatty acids of microalgae: Diversity and applications. Rev. Environ. Sci. Bio/Technol. 2021;20:515–547. doi: 10.1007/s11157-021-09571-3. - DOI
-
- Rizwan M., Mujtaba G., Ahmed S., Lee K., Rashid N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018;92:394–404. doi: 10.1016/j.rser.2018.04.034. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

