Iron in Porphyrias: Friend or Foe?
- PMID: 35204362
- PMCID: PMC8870839
- DOI: 10.3390/diagnostics12020272
Iron in Porphyrias: Friend or Foe?
Abstract
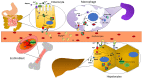
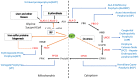
Iron is a trace element that is important for many vital processes, including oxygen transport, oxidative metabolism, cellular proliferation, and catalytic reactions. Iron supports these functions mainly as part of the heme molecule. Heme synthesis is an eight-step process which, when defective at the level of one of the eight enzymes involved, can cause the development of a group of diseases, either inherited or acquired, called porphyrias. Despite the strict link between iron and heme, the role of iron in the different types of porphyrias, particularly as a risk factor for disease development/progression or as a potential therapeutic target or molecule, is still being debated, since contrasting results have emerged from clinical observations, in vitro studies and animal models. In this review we aim to deepen such aspects by drawing attention to the current evidence on the role of iron in porphyrias and its potential implication. Testing for iron status and its metabolic pathways through blood tests, imaging techniques or genetic studies on patients affected by porphyrias can provide additional diagnostic and prognostic value to the clinical care, leading to a more tailored and effective management.
Keywords: anemia; heme; hepcidin; iron; porphyrias.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

