OCT-Guided Surgery for Gliomas: Current Concept and Future Perspectives
- PMID: 35204427
- PMCID: PMC8871129
- DOI: 10.3390/diagnostics12020335
OCT-Guided Surgery for Gliomas: Current Concept and Future Perspectives
Abstract
Optical coherence tomography (OCT) has been recently suggested as a promising method to obtain in vivo and real-time high-resolution images of tissue structure in brain tumor surgery. This review focuses on the basics of OCT imaging, types of OCT images and currently suggested OCT scanner devices and the results of their application in neurosurgery. OCT can assist in achieving intraoperative precision identification of tumor infiltration within surrounding brain parenchyma by using qualitative or quantitative OCT image analysis of scanned tissue. OCT is able to identify tumorous tissue and blood vessels detection during stereotactic biopsy procedures. The combination of OCT with traditional imaging such as MRI, ultrasound and 5-ALA fluorescence has the potential to increase the safety and accuracy of the resection. OCT can improve the extent of resection by offering the direct visualization of tumor with cellular resolution when using microscopic OCT contact probes. The theranostic implementation of OCT as a part of intelligent optical diagnosis and automated lesion localization and ablation could achieve high precision, automation and intelligence in brain tumor surgery. We present this review for the increase of knowledge and formation of critical opinion in the field of OCT implementation in brain tumor surgery.
Keywords: brain imaging; brain tumor; intraoperative imaging; minimally invasive theranostics; neurosurgical guidance; optical coherence tomography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



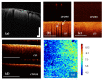
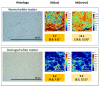
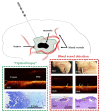
References
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

