Peripartum Cardiomyopathy: Diagnostic and Prognostic Value of Cardiac Magnetic Resonance in the Acute Stage
- PMID: 35204469
- PMCID: PMC8871076
- DOI: 10.3390/diagnostics12020378
Peripartum Cardiomyopathy: Diagnostic and Prognostic Value of Cardiac Magnetic Resonance in the Acute Stage
Abstract
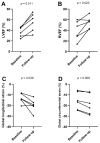
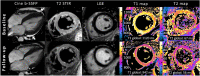
This study aimed to evaluate the diagnostic and prognostic value of cardiac magnetic resonance in acute peripartum cardiomyopathy (PPCM). A total of 17 patients with PPCM in the acute stage and 15 healthy controls were retrospectively analyzed regarding myocardial function, edema, late gadolinium enhancement (LGE), and T1 and T2 mappings (T1, T2). Echocardiographic follow-ups were performed. Functional recovery was defined as a left ventricular ejection fraction (LVEF) of ≥50%. Patients with PPCM displayed biventricular dysfunction with reduced myocardial strain parameters and left ventricular and atrial dilatation, as well as diffuse myocardial edema (T2 signal intensity ratio: 2.10 ± 0.34 vs. 1.58 ± 0.21, p < 0.001; T1: 1070 ± 51 ms vs. 980 ± 28 ms, p = 0.001; T2: 63 ± 5 ms vs. 53 ± 2 ms, p < 0.001). Visual myocardial edema was present in 10 patients (59%). LGE was positive in 2 patients (12%). A total of 13 patients (76%) showed full LVEF recovery. The absence of visual myocardial edema and impairment of strain parameters were associated with delayed LVEF recovery. Multivariable Cox regression analysis revealed global longitudinal strain as an independent prognostic factor for LVEF recovery. In conclusion, biventricular systolic dysfunction with diffuse myocardial edema seems to be present in acute PPCM. Myocardial edema and strain may have prognostic value for LVEF recovery.
Keywords: cardiac magnetic resonance imaging; heart failure; mapping; myocardial edema; peripartum cardiomyopathy; pregnancy; strain.
Conflict of interest statement
U.A. disclosed speaker fees received from Siemens Healthineers in 2018. J.A.L. disclosed speaker fees from Bayer HealthCare and Philips Healthcare.
Figures


References
-
- Bauersachs J., König T., van der Meer P., Petrie M.C., Hilfiker-Kleiner D., Mbakwem A., Hamdan R., Jackson A.M., Forsyth P., de Boer R.A., et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2019;21:827–843. doi: 10.1002/ejhf.1493. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

