Evaluation and Modelling of the Performance of an Automated SARS-CoV-2 Antigen Assay According to Sample Type, Target Population and Epidemic Trends
- PMID: 35204538
- PMCID: PMC8871059
- DOI: 10.3390/diagnostics12020447
Evaluation and Modelling of the Performance of an Automated SARS-CoV-2 Antigen Assay According to Sample Type, Target Population and Epidemic Trends
Abstract
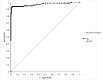
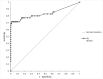
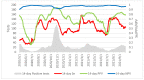
The Lumipulse® G SARS-CoV-2 Ag assay performance was evaluated on prospectively collected saliva and nasopharyngeal swabs (NPS) of recently ill in- and outpatients and according to the estimated viral load. Performances were calculated using RT-PCR positive NPS from patients with symptoms ≤ 7 days and RT-PCR negative NPS as gold standard. In addition, non-selected positive NPS were analyzed to assess the performances on various viral loads. This assay yielded a sensitivity of 93.1% on NPS and 71.4% on saliva for recently ill patients. For NPS with a viral load > 103 RNA copies/mL, sensitivity was 96.4%. A model established on our daily routine showed fluctuations of the performances depending on the epidemic trends but an overall good negative predictive value. Lumipulse® G SARS-CoV-2 assay yielded good performance for an automated antigen detection assay on NPS. Using it for the detection of recently ill patients or to screen high-risk patients could be an interesting alternative to the more expensive RT-PCR.
Keywords: COVID-19; SARS-CoV-2; assay; diagnostic; model; test.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- World Health Organization . Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection—Interim Guidance. World Health Organization; Geneva, Switzerland: 2021.
-
- European Centre for Disease Prevention and Control . Options for the Use of Rapid Antigen Tests for COVID-19 in the EU/EEA and the UK. European Centre for Disease Prevention and Control; Solna, Sweden: 2020.
-
- Hirotsu Y., Maejima M., Shibusawa M., Nagakubo Y., Hosaka K., Amemiya K., Sueki H., Hayakawa M., Mochizuki H., Tsutsui T., et al. Comparison of Automated SARS-CoV-2 Antigen Test for COVID-19 Infection with Quantitative RT-PCR Using 313 Nasopharyngeal Swabs, Including from Seven Serially Followed Patients. Int. J. Infect. Dis. 2020;99:397–402. doi: 10.1016/j.ijid.2020.08.029. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

