Lactococcus lactis, an Attractive Cell Factory for the Expression of Functional Membrane Proteins
- PMID: 35204681
- PMCID: PMC8961550
- DOI: 10.3390/biom12020180
Lactococcus lactis, an Attractive Cell Factory for the Expression of Functional Membrane Proteins
Abstract
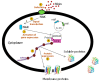
Membrane proteins play key roles in most crucial cellular processes, ranging from cell-to-cell communication to signaling processes. Despite recent improvements, the expression of functionally folded membrane proteins in sufficient amounts for functional and structural characterization remains a challenge. Indeed, it is still difficult to predict whether a protein can be overproduced in a functional state in some expression system(s), though studies of high-throughput screens have been published in recent years. Prokaryotic expression systems present several advantages over eukaryotic ones. Among them, Lactococcus lactis (L. lactis) has emerged in the last two decades as a good alternative expression system to E. coli. The purpose of this chapter is to describe L. lactis and its tightly inducible system, NICE, for the effective expression of membrane proteins from both prokaryotic and eukaryotic origins.
Keywords: Lactococcus lactis; NICE system; membrane proteins.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

