Long-Term Varicella Zoster Virus Immunity in Paediatric Liver Transplant Patients Can Be Achieved by Booster Vaccinations-A Single-Centre, Retrospective, Observational Analysis
- PMID: 35204851
- PMCID: PMC8870030
- DOI: 10.3390/children9020130
Long-Term Varicella Zoster Virus Immunity in Paediatric Liver Transplant Patients Can Be Achieved by Booster Vaccinations-A Single-Centre, Retrospective, Observational Analysis
Abstract
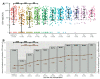
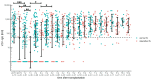
Varicella is one of the most common vaccine-preventable infections after paediatric solid organ transplantation; thus, vaccination offers simple and cheap protection. However, children with liver disease often progress to liver transplantation (LT) before they reach the recommended vaccination age. As a live vaccine, varicella zoster virus (VZV) vaccination after transplantation is controversial; however, many case series demonstrate that vaccination may be safe and effective in paediatric liver transplant recipients. Only limited data exists describing long-term vaccination response in such immunocompromised patients. We investigated retrospectively vaccination response in paediatric patients before and after transplantation and describe long-term immunity over ten years, including the influence of booster-vaccinations. In this retrospective, single-centre study, 458 LT recipients were analysed between September 2004 and June 2021. Of these, 53 were re-transplantations. Patients with no available vaccination records and with a history of post-transplant lymphoproliferative disease, after hematopoietic stem cell transplantation and clinical chickenpox were excluded from this analysis (n = 198). In total, data on 207 children with a median annual follow-up of 6.2 years was available: 95 patients (45.9%) were unvaccinated prior to LT. Compared to healthy children, the response to vaccination, measured by seroconversion, is weaker in children with liver disease: almost 70% after one vaccination and 93% after two vaccinations. One year after transplantation, the mean titres and the number of children with protective antibody levels (VZV IgG ≥ 50 IU/L) decreased from 77.5% to 41.3%. Neither diagnosis, gender, nor age were predictors of vaccination response. Booster-vaccination was recommended for children after seroreversion using annual titre measurements and led to a significant increase in mean titre and number of protected children. Response to vaccination shows no difference from monotherapy with a calcineurin inhibitor to intensified immunosuppression by adding prednisolone or mycophenolate mofetil. Children with liver disease show weaker seroconversion rates to VZV vaccination compared to healthy children. Therefore, VZV-naïve children should receive basic immunization with two vaccine doses as well as those vaccinated only once before transplantation. An average of 2-3 vaccine doses are required in order to achieve a long-term seroconversion and protective antibody levels in 95% of children.
Keywords: VZV; chickenpox; chronic liver disease; immunization; immunosuppression; paediatric liver transplantation; vaccination; varicella.
Conflict of interest statement
The authors declare there is no conflict of interest.
Figures

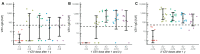
Similar articles
-
Diminished measles immunity after paediatric liver transplantation-A retrospective, single-centre, cross-sectional analysis.PLoS One. 2024 Feb 5;19(2):e0296653. doi: 10.1371/journal.pone.0296653. eCollection 2024. PLoS One. 2024. PMID: 38315673 Free PMC article.
-
Boosting the VZV-Specific Memory B and T Cell Response to Prevent Herpes Zoster After Kidney Transplantation.Front Immunol. 2022 Jul 22;13:927734. doi: 10.3389/fimmu.2022.927734. eCollection 2022. Front Immunol. 2022. PMID: 35935972 Free PMC article.
-
Long-term study showed that vaccination protected paediatric renal transplant recipients from life-threatening varicella zoster virus.Acta Paediatr. 2018 Dec;107(12):2185-2192. doi: 10.1111/apa.14375. Epub 2018 May 25. Acta Paediatr. 2018. PMID: 29706010 Free PMC article.
-
[Vaccination].Klin Padiatr. 2001 Sep;213 Suppl 1:A77-83. doi: 10.1055/s-2001-17503. Klin Padiatr. 2001. PMID: 11577366 Review. German.
-
Varicella vaccination in Japan: necessity of implementing a routine vaccination program.J Infect Chemother. 2013 Apr;19(2):188-95. doi: 10.1007/s10156-013-0577-x. Epub 2013 Mar 13. J Infect Chemother. 2013. PMID: 23483311 Review.
Cited by
-
Advancements in Vaccine Strategies for Chronic Liver Disease Patients: Navigating Post-COVID Challenges and Opportunities.Vaccines (Basel). 2024 Feb 15;12(2):197. doi: 10.3390/vaccines12020197. Vaccines (Basel). 2024. PMID: 38400180 Free PMC article. Review.
-
A quick algorithmic review on management of viral infectious diseases in pediatric solid organ transplant recipients.Front Pediatr. 2023 Sep 4;11:1252495. doi: 10.3389/fped.2023.1252495. eCollection 2023. Front Pediatr. 2023. PMID: 37732007 Free PMC article. Review.
-
Diminished measles immunity after paediatric liver transplantation-A retrospective, single-centre, cross-sectional analysis.PLoS One. 2024 Feb 5;19(2):e0296653. doi: 10.1371/journal.pone.0296653. eCollection 2024. PLoS One. 2024. PMID: 38315673 Free PMC article.
References
-
- Leiskau C., Junge N., Pfister E., Goldschmidt I., Mutschler F., Laue T., Ohlendorf J., Nasser H., Beneke J., Richter N., et al. Recipient-Specific Risk Factors Impairing Patient and Graft Outcome after Pediatric Liver Transplantation—Analysis of 858 Transplantations in 38 Years. Children. 2021;8:641. doi: 10.3390/children8080641. - DOI - PMC - PubMed
-
- Farmer D.G., Venick R.S., McDiarmid S.V., Ghobrial R.M., Gordon S.A., Yersiz H., Hong J., Candell L., Cholakians A., Wozniak L., et al. Predictors of outcomes after pediatric liver transplantation: An analysis of more than 800 cases performed at a single institution. J. Am. Coll. Surg. 2007;204:904–914. doi: 10.1016/j.jamcollsurg.2007.01.061. - DOI - PubMed
-
- Feldman A.G., Beaty B.L., Curtis D., Juarez-Colunga E., Kempe A. Incidence of Hospitalization for Vaccine-Preventable Infections in Children Following Solid Organ Transplant and Associated Morbidity, Mortality, and Costs. JAMA Pediatr. 2019;173:260–268. doi: 10.1001/jamapediatrics.2018.4954. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

