Procollagen C-Endopeptidase Enhancer 2 Secreted by Tonsil-Derived Mesenchymal Stem Cells Increases the Oxidative Burst of Promyelocytic HL-60 Cells
- PMID: 35205121
- PMCID: PMC8869569
- DOI: 10.3390/biology11020255
Procollagen C-Endopeptidase Enhancer 2 Secreted by Tonsil-Derived Mesenchymal Stem Cells Increases the Oxidative Burst of Promyelocytic HL-60 Cells
Abstract
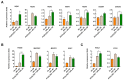
Reactive oxygen species (ROS) generated by neutrophils provide a frontline defence against invading pathogens. We investigated the supportive effect of tonsil-derived mesenchymal stem cells (TMSCs) on ROS generation from neutrophils using promyelocytic HL-60 cells. Methods: Differentiated HL-60 (dHL-60) cells were cocultured with TMSCs isolated from 25 independent donors, and ROS generation in dHL-60 cells was measured using luminescence. RNA sequencing and real-time PCR were performed to identify the candidate genes of TMSCs involved in augmenting the oxidative burst of dHL-60 cells. Transcriptome analysis of TMSCs derived from 25 independent donors revealed high levels of procollagen C-endopeptidase enhancer 2 (PCOLCE2) in TMSCs, which were highly effective in potentiating ROS generation in dHL-60 cells. In addition, PCOLCE2 knockdown in TMSCs abrogated TMSC-induced enhancement of ROS production in dHL-60 cells, indicating that TMSCs increased the oxidative burst in dHL-60 cells via PCOLCE2. Furthermore, the direct addition of recombinant PCOLCE2 protein increased ROS production in dHL-60 cells. These results suggest that PCOLCE2 secreted by TMSCs may be used as a therapeutic candidate to enhance host defences by increasing neutrophil oxidative bursts. PCOLCE2 levels in TMSCs could be used as a marker to select TMSCs exhibiting high efficacy for enhancing neutrophil oxidative bursts.
Keywords: donor variation; neutrophil; oxidative burst; procollagen C-endopeptidase enhancer 2; tonsil derived mesenchymal stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

