The Transcription Factor FgAtrR Regulates Asexual and Sexual Development, Virulence, and DON Production and Contributes to Intrinsic Resistance to Azole Fungicides in Fusarium graminearum
- PMID: 35205191
- PMCID: PMC8869466
- DOI: 10.3390/biology11020326
The Transcription Factor FgAtrR Regulates Asexual and Sexual Development, Virulence, and DON Production and Contributes to Intrinsic Resistance to Azole Fungicides in Fusarium graminearum
Abstract
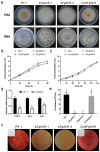
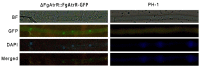
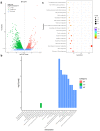
Fusarium graminearum is the predominant causal agent of cereal Fusarium head blight disease (FHB) worldwide. The application of chemical fungicides such as azole antifungals is still the primary method for FHB control. However, to date, our knowledge of transcriptional regulation in the azole resistance of F. graminearum is quite limited. In this study, we identified and functionally characterized a Zn(II)2-Cys6 transcription factor FgAtrR in F. graminearum. We constructed a FgAtrR deletion mutant and found that deletion of FgAtrR resulted in faster radial growth with serious pigmentation defects, significantly reduced conidial production, and an inability to form perithecia. The pathogenicity of the ΔFgAtrR mutant on wheat spikes and corn silks was severely impaired with reduced deoxynivalenol production, while the tolerance to prochloraz and propiconazole of the deletion mutant was also significantly decreased. RNA-seq indicated that many metabolic pathways were affected by the deletion of FgAtrR. Importantly, FgAtrR could regulate the expression of the FgCYP51A and ABC transporters, which are the main contributors to azole resistance. These results demonstrated that FgAtrR played essential roles in asexual and sexual development, DON production, and pathogenicity, and contributed to intrinsic resistance to azole fungicides in F. graminearum. This study will help us improve the understanding of the azole resistance mechanism in F. graminearum.
Keywords: Fusarium head blight; fungicide resistance; mycotoxin; plant pathogen; transcriptional regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
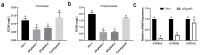
Similar articles
-
The Non-Histone Protein FgNhp6 Is Involved in the Regulation of the Development, DON Biosynthesis, and Virulence of Fusarium graminearum.Pathogens. 2024 Jul 16;13(7):592. doi: 10.3390/pathogens13070592. Pathogens. 2024. PMID: 39057819 Free PMC article.
-
Toxicity and action mechanisms of silver nanoparticles against the mycotoxin-producing fungus Fusarium graminearum.J Adv Res. 2021 Sep 17;38:1-12. doi: 10.1016/j.jare.2021.09.006. eCollection 2022 May. J Adv Res. 2021. PMID: 35572400 Free PMC article.
-
The Endoplasmic Reticulum Cargo Receptor FgErv14 Regulates DON Production, Growth and Virulence in Fusarium graminearum.Life (Basel). 2022 May 27;12(6):799. doi: 10.3390/life12060799. Life (Basel). 2022. PMID: 35743830 Free PMC article.
-
Transcriptomics of cereal-Fusarium graminearum interactions: what we have learned so far.Mol Plant Pathol. 2018 Mar;19(3):764-778. doi: 10.1111/mpp.12561. Epub 2017 Jun 7. Mol Plant Pathol. 2018. PMID: 28411402 Free PMC article. Review.
-
Recent advances in genes involved in secondary metabolite synthesis, hyphal development, energy metabolism and pathogenicity in Fusarium graminearum (teleomorph Gibberella zeae).Biotechnol Adv. 2014 Mar-Apr;32(2):390-402. doi: 10.1016/j.biotechadv.2013.12.007. Epub 2014 Jan 2. Biotechnol Adv. 2014. PMID: 24389085 Review.
Cited by
-
Effects of Fungicides and Nontarget Pesticides on Accumulation of the Mycotoxin Deoxynivlenol in Wheat.Toxics. 2023 Sep 10;11(9):768. doi: 10.3390/toxics11090768. Toxics. 2023. PMID: 37755778 Free PMC article.
-
PWWP domain-containing protein Crf4-3 specifically modulates fungal azole susceptibility by regulating sterol C-14 demethylase ERG11.mSphere. 2025 Jan 28;10(1):e0070324. doi: 10.1128/msphere.00703-24. Epub 2024 Dec 13. mSphere. 2025. PMID: 39670730 Free PMC article.
-
The Non-Histone Protein FgNhp6 Is Involved in the Regulation of the Development, DON Biosynthesis, and Virulence of Fusarium graminearum.Pathogens. 2024 Jul 16;13(7):592. doi: 10.3390/pathogens13070592. Pathogens. 2024. PMID: 39057819 Free PMC article.
-
Transcription factor-dependent regulatory networks of sexual reproduction in Fusarium graminearum.mBio. 2025 Jan 8;16(1):e0303024. doi: 10.1128/mbio.03030-24. Epub 2024 Nov 26. mBio. 2025. PMID: 39589130 Free PMC article.
-
Voriconazole Treatment Induces a Conserved Sterol/Pleiotropic Drug Resistance Regulatory Network, including an Alternative Ergosterol Biosynthesis Pathway, in the Clinically Important FSSC Species, Fusarium keratoplasticum.J Fungi (Basel). 2022 Oct 12;8(10):1070. doi: 10.3390/jof8101070. J Fungi (Basel). 2022. PMID: 36294635 Free PMC article.
References
-
- Dweba C.C., Figlan S., Shimelis H.A., Motaung T.E., Sydenham S., Mwadzingeni L., Tsilo T.J. Fusarium Head Blight of Wheat: Pathogenesis and Control Strategies. Crop Prot. 2017;91:114–122. doi: 10.1016/j.cropro.2016.10.002. - DOI
-
- West J.S., Holdgate S., Townsend J.A., Edwards S.G., Jennings P., Fitt B.D.L. Impacts of Changing Climate and Agronomic Factors on Fusarium Ear Blight of Wheat in the UK. Fungal Ecol. 2012;5:53–61. doi: 10.1016/j.funeco.2011.03.003. - DOI
-
- Chakraborty S., Newton A.C. Climate Change, Plant Diseases and Food Security: An Overview. Plant Pathol. 2011;60:2–14. doi: 10.1111/j.1365-3059.2010.02411.x. - DOI
-
- Ehling G., Cockburn A., Snowdon P., Buschhaus H. The Significance of the Fusarium Toxin Deoxynivalenol (DON) for Human and Animal Health. Cereal Res. Commun. 1997;25:443–447. doi: 10.1007/BF03543749. - DOI
-
- Ji F., He D., Olaniran A.O., Mokoena M.P., Xu J., Shi J. Occurrence, Toxicity, Production and Detection of Fusarium Mycotoxin: A Review. Food Prod. Process. Nutr. 2019;1:6. doi: 10.1186/s43014-019-0007-2. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

