MtWRP1, a Novel Fabacean Specific Gene, Regulates Root Nodulation and Plant Growth in Medicago truncatula
- PMID: 35205237
- PMCID: PMC8871812
- DOI: 10.3390/genes13020193
MtWRP1, a Novel Fabacean Specific Gene, Regulates Root Nodulation and Plant Growth in Medicago truncatula
Abstract
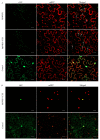
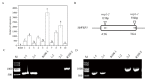
Fabaceans symbiotically interact with nitrogen-fixing rhizobacteria to form root nodules. Some fabacean specific proteins play important roles in the symbiosis. WRKY-related Protein (WRP) is a novel fabacean specific protein, whose functions have not been well characterized. In this study, MtWRP1 was functionally characterized in Medicago truncatula. It contains a WRKY domain at C-terminal and a novel transmembrane (TM) domain at N-terminal, and its WRKY domain was highly similar to the N-terminal WRKY domain of the group I WRKY proteins. The TM domain was highly homologous to the eukaryotic cytochrome b561 (Cytb561) proteins from birds. Subcellular localization revealed that MtWRP1 was targeted to the Golgi apparatus through the novel TM domain. MtWRP1 was highly expressed in roots and nodules, suggesting its possible roles in the regulation of root growth and nodulation. Both MtWRP1-overexpression transgenic M. truncatula and MtWRP1 mutants showed altered root nodulation and plant growth performance. Specifically, the formation of root nodules was significantly reduced in the absence of MtWRP1. These results demonstrated that MtWRP1 plays critical roles in root nodulation and plant growth.
Keywords: Medicago truncatula; MtWRP1; N-terminal transmembrane domain; nodulation; plant growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Peix A., Ramírez-Bahena M.H., Velázquez E., Bedmar E.J. Bacterial Associations with Legumes. Crit. Rev. Plant Sci. 2014;34:17–42. doi: 10.1080/07352689.2014.897899. - DOI
-
- Peoples M.B., Ladha D.F., Herridge J.K. Biological Nitrogen Fixation: An Efficient Source of Nitrogen for Sustainable Agricultural Production? Plant Soil. 1995;174:3–28. doi: 10.1007/BF00032239. - DOI
-
- Koskey G., Mburu S.W., Njeru E.M., Kimiti J.M., Ombori O., Maingi J.M. Potential of Native Rhizobia in Enhancing Nitrogen Fixation and Yields of Climbing Beans (Phaseolus vulgaris L.) in Contrasting Environments of Eastern Kenya. Front. Plant Sci. 2017;8:443. doi: 10.3389/fpls.2017.00443. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

