Initiator-Directed Transcription: Fission Yeast Nmtl Initiator Directs Preinitiation Complex Formation and Transcriptional Initiation
- PMID: 35205301
- PMCID: PMC8871863
- DOI: 10.3390/genes13020256
Initiator-Directed Transcription: Fission Yeast Nmtl Initiator Directs Preinitiation Complex Formation and Transcriptional Initiation
Abstract
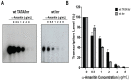
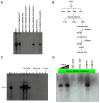
The initiator element is a core promoter element encompassing the transcription start site, which is found in yeast, Drosophila, and human promoters. This element is observed in TATA-less promoters. Several studies have defined transcription factor requirements and additional cofactors that are needed for transcription initiation of initiator-containing promoters. However, those studies have been performed with additional core promoters in addition to the initiator. In this work, we have defined the pathway of preinitiation complex formation on the fission yeast nmt1 gene promoter, which contains a functional initiator with striking similarity to the initiator of the human dihydrofolate reductase (hDHFR) gene and to the factor requirement for transcription initiation of the nmt1 gene promoter. The results show that the nmt1 gene promoter possesses an initiator encompassing the transcription start site, and several conserved base positions are required for initiator function. A preinitiation complex formation on the nmt1 initiator can be started by TBP/TFIIA or TBP/TFIIB, but not TBP alone, and afterwards follows the same pathway as preinitiation complex formation on TATA-containing promoters. Transcription initiation is dependent on the general transcription factors TBP, TFIIB, TFIIE, TFIIF, TFIIH, RNA polymerase II, Mediator, and a cofactor identified as transcription cofactor for initiator function (TCIF), which is a high-molecular-weight protein complex of around 500 kDa. However, the TAF subunits of TFIID were not required for the nmt1 initiator transcription, as far as we tested. We also demonstrate that other initiators of the nmt1/hDHFR family can be transcribed in fission yeast whole-cell extracts.
Keywords: RNA polymerase II; Schizosaccharomyces pombe; general transcription factors (GTFs); initiator; transcription.
Conflict of interest statement
The authors declare that there are no conflict of interest with the contents of this article.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

