Maternal Uniparental Isodisomy of Chromosome 4 and 8 in Patients with Retinal Dystrophy: SRD5A3-Congenital Disorders of Glycosylation and RP1-Related Retinitis Pigmentosa
- PMID: 35205402
- PMCID: PMC8872353
- DOI: 10.3390/genes13020359
Maternal Uniparental Isodisomy of Chromosome 4 and 8 in Patients with Retinal Dystrophy: SRD5A3-Congenital Disorders of Glycosylation and RP1-Related Retinitis Pigmentosa
Abstract
Purpose: Uniparental disomy (UPD) is a rare chromosomal abnormality. We performed whole-exosome sequencing (WES) in cases of early-onset retinal dystrophy and identified two cases likely caused by UPD. Herein, we report these two cases and attempt to clarify the clinical picture of retinal dystrophies caused by UPD.
Methods: WES analysis was performed for two patients and their parents, who were not consanguineous. Functional analysis was performed in cases suspected of congenital disorders of glycosylation (CDG). We obtained clinical case data and reviewed the literature.
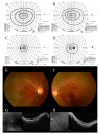
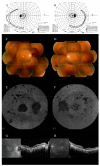
Results: In case 1, a novel c.57G>C, p.(Trp19Cys) variant in SRD5A3 was detected homozygously. Genetic analysis suggested a maternal UPD on chromosome 4, and functional analysis confirmed CDG. Clinical findings showed early-onset retinal dystrophy, intellectual disability, and epilepsy. In case 2, an Alu insertion (c.4052_4053ins328, p.[Tyr1352Alafs]) in RP1 was detected homozygously. Maternal UPD on chromosome 8 was suspected. The clinical picture was consistent with RP1-related retinitis pigmentosa. Although the clinical features of retinal dystrophy by UPD may vary, most cases present with childhood onset.
Conclusions: There have been limited reports of retinal dystrophy caused by UPD, suggesting that it is rare. Genetic counseling may be encouraged in pediatric cases of retinal dystrophy.
Keywords: RP1-related retinitis pigmentosa; SRD5A3 gene; congenital disorders of glycosylation; retinal dystrophy; uniparental isodisomy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Mutirangura A., Greenberg F., Butler M.G., Malcolm S., Nicholls R.D., Chakravarti A., Ledbetter D.H. Multiplex PCR of Three Dinucleotide Repeats in the Prader-Willi/Angelman Critical Region (15q11-Q13): Molecular Diagnosis and Mechanism of Uniparental Disomy. Hum. Mol. Genet. 1993;2:143–151. doi: 10.1093/hmg/2.2.143. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

