DKK3, Downregulated in Invasive Epithelial Ovarian Cancer, Is Associated with Chemoresistance and Enhanced Paclitaxel Susceptibility via Inhibition of the β-Catenin-P-Glycoprotein Signaling Pathway
- PMID: 35205672
- PMCID: PMC8870560
- DOI: 10.3390/cancers14040924
DKK3, Downregulated in Invasive Epithelial Ovarian Cancer, Is Associated with Chemoresistance and Enhanced Paclitaxel Susceptibility via Inhibition of the β-Catenin-P-Glycoprotein Signaling Pathway
Abstract
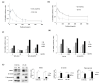
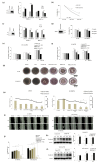
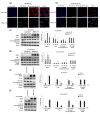
Dickkopf-3 (DKK3), a tumor suppressor, is frequently downregulated in various cancers. However, the role of DKK3 in ovarian cancer has not been evaluated. This study aimed to assess aberrant DKK3 expression and its role in epithelial ovarian carcinoma. DKK3 expression was assessed using immunohistochemistry with tissue blocks from 82 patients with invasive carcinoma, and 15 normal, 19 benign, and 10 borderline tumors as controls. Survival data were analyzed using Kaplan-Meier and Cox regression analysis. Paclitaxel-resistant cells were established using TOV-21G and OV-90 cell lines. Protein expression was assessed using Western blotting and immunofluorescence analysis. Cell viability was assessed using the MT assay and 3D-spheroid assay. Cell migration was determined using a migration assay. DKK3 was significantly downregulated in invasive carcinoma compared to that in normal, benign, and borderline tumors. DKK3 loss occurred in 56.1% invasive carcinomas and was significantly associated with disease-free survival and chemoresistance in serous adenocarcinoma. DKK3 was lost in paclitaxel-resistant cells, while β-catenin and P-glycoprotein were upregulated. Exogenous secreted DKK3, incorporated by cells, enhanced anti-tumoral effect and paclitaxel susceptibility in paclitaxel-resistant cells, and reduced the levels of active β-catenin and its downstream P-glycoprotein, suggesting that DKK3 can be used as a therapeutic for targeting paclitaxel-resistant cancer.
Keywords: DKK3; P-glycoprotein; ovarian cancer; paclitaxel resistance; β-catenin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Class III β-tubulin overexpression in ovarian clear cell and serous carcinoma as a maker for poor overall survival after platinum/taxane chemotherapy and sensitivity to patupilone.Am J Obstet Gynecol. 2013 Jul;209(1):62.e1-9. doi: 10.1016/j.ajog.2013.04.017. Epub 2013 Apr 10. Am J Obstet Gynecol. 2013. PMID: 23583215
-
Dickkopf-3 (Dkk3) induces apoptosis in cisplatin-resistant lung adenocarcinoma cells via the Wnt/β-catenin pathway.Oncol Rep. 2015 Mar;33(3):1097-106. doi: 10.3892/or.2014.3704. Epub 2014 Dec 30. Oncol Rep. 2015. PMID: 25573172
-
Dkk3, downregulated in cervical cancer, functions as a negative regulator of beta-catenin.Int J Cancer. 2009 Jan 15;124(2):287-97. doi: 10.1002/ijc.23913. Int J Cancer. 2009. PMID: 19003969
-
Wnt signalling in human breast cancer: expression of the putative Wnt inhibitor Dickkopf-3 (DKK3) is frequently suppressed by promoter hypermethylation in mammary tumours.Breast Cancer Res. 2008;10(5):R82. doi: 10.1186/bcr2151. Epub 2008 Sep 30. Breast Cancer Res. 2008. PMID: 18826564 Free PMC article.
-
DNA methylation of DKK3 modulates docetaxel chemoresistance in human nonsmall cell lung cancer cell.Cancer Biother Radiopharm. 2015 Mar;30(2):100-6. doi: 10.1089/cbr.2014.1797. Cancer Biother Radiopharm. 2015. PMID: 25760729
Cited by
-
The Multifaceted Role of Human Dickkopf-3 (DKK-3) in Development, Immune Modulation and Cancer.Cells. 2023 Dec 29;13(1):75. doi: 10.3390/cells13010075. Cells. 2023. PMID: 38201279 Free PMC article. Review.
-
Human endogenous retrovirus-K envelope protein is aberrantly expressed in serous ovarian cancer and promotes chemosensitivity via NF-κB/P-glycoprotein pathway inhibition.J Ovarian Res. 2025 Jul 4;18(1):146. doi: 10.1186/s13048-025-01722-2. J Ovarian Res. 2025. PMID: 40616136 Free PMC article.
-
Lumbrokinase, a Fibrinolytic Enzyme, Prevents Intra-Abdominal Adhesion by Inhibiting the Migrative and Adhesive Activities of Fibroblast via Attenuation of the AP-1/ICAM-1 Signaling Pathway.Biomed Res Int. 2023 Jan 12;2023:4050730. doi: 10.1155/2023/4050730. eCollection 2023. Biomed Res Int. 2023. PMID: 36685669 Free PMC article.
-
DKK3 and SERPINB5 as novel serum biomarkers for gastric cancer: facilitating the development of risk prediction models for gastric cancer.Front Oncol. 2025 Mar 31;15:1536491. doi: 10.3389/fonc.2025.1536491. eCollection 2025. Front Oncol. 2025. PMID: 40231256 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

