Early Neutrophilia Marked by Aerobic Glycolysis Sustains Host Metabolism and Delays Cancer Cachexia
- PMID: 35205709
- PMCID: PMC8870098
- DOI: 10.3390/cancers14040963
Early Neutrophilia Marked by Aerobic Glycolysis Sustains Host Metabolism and Delays Cancer Cachexia
Abstract
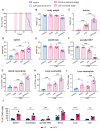
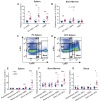
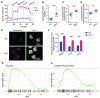
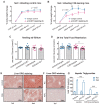
An elevated neutrophil-lymphocyte ratio negatively predicts the outcome of patients with cancer and is associated with cachexia, the terminal wasting syndrome. Here, using murine model systems of colorectal and pancreatic cancer we show that neutrophilia in the circulation and multiple organs, accompanied by extramedullary hematopoiesis, is an early event during cancer progression. Transcriptomic and metabolic assessment reveals that neutrophils in tumor-bearing animals utilize aerobic glycolysis, similar to cancer cells. Although pharmacological inhibition of aerobic glycolysis slows down tumor growth in C26 tumor-bearing mice, it precipitates cachexia, thereby shortening the overall survival. This negative effect may be explained by our observation that acute depletion of neutrophils in pre-cachectic mice impairs systemic glucose homeostasis secondary to altered hepatic lipid processing. Thus, changes in neutrophil number, distribution, and metabolism play an adaptive role in host metabolic homeostasis during cancer progression. Our findings provide insight into early events during cancer progression to cachexia, with implications for therapy.
Keywords: aerobic glycolysis; cachexia; cancer; host; metabolism; neutrophils.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Dusselier M., Deluche E., Delacourt N., Ballouhey J., Egenod T., Melloni B., Vergnenegre C., Veillon R., Vergnenegre A. Neutrophil-to-lymphocyte ratio evolution is an independent predictor of early progression of second-line nivolumab-treated patients with advanced non-small-cell lung cancers. PLoS ONE. 2019;14:e0219060. doi: 10.1371/journal.pone.0219060. - DOI - PMC - PubMed
-
- Kulbe H., Thompson R., Wilson J.L., Robinson S., Hagemann T., Fatah R., Gould D., Ayhan A., Balkwill F. The Inflammatory Cytokine Tumor Necrosis Factor-α Generates an Autocrine Tumor-Promoting Network in Epithelial Ovarian Cancer Cells. Cancer Res. 2007;67:585–592. doi: 10.1158/0008-5472.CAN-06-2941. - DOI - PMC - PubMed
Grants and funding
- MC_UU_12022/8/MRC_/Medical Research Council/United Kingdom
- A24995/CRUK_/Cancer Research UK/United Kingdom
- MC_UU_12022/1/MRC_/Medical Research Council/United Kingdom
- C42738/A24868/CRUK_/Cancer Research UK/United Kingdom
- 204622/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- P30 CA045508/CA/NCI NIH HHS/United States
- 24868/CRUK_/Cancer Research UK/United Kingdom
- MC_UU_12022/6/MRC_/Medical Research Council/United Kingdom
- Clinical Lecturer/AMS_/Academy of Medical Sciences/United Kingdom
- 24995/CRUK_/Cancer Research UK/United Kingdom
- 15678/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources

