Dissecting Tumor Growth: The Role of Cancer Stem Cells in Drug Resistance and Recurrence
- PMID: 35205721
- PMCID: PMC8869911
- DOI: 10.3390/cancers14040976
Dissecting Tumor Growth: The Role of Cancer Stem Cells in Drug Resistance and Recurrence
Abstract
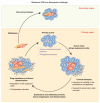
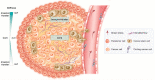
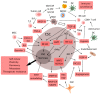
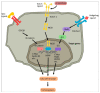
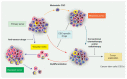
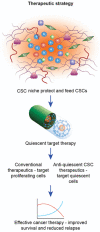
Emerging evidence suggests that a small subpopulation of cancer stem cells (CSCs) is responsible for initiation, progression, and metastasis cascade in tumors. CSCs share characteristics with normal stem cells, i.e., self-renewal and differentiation potential, suggesting that they can drive cancer progression. Consequently, targeting CSCs to prevent tumor growth or regrowth might offer a chance to lead the fight against cancer. CSCs create their niche, a specific area within tissue with a unique microenvironment that sustains their vital functions. Interactions between CSCs and their niches play a critical role in regulating CSCs' self-renewal and tumorigenesis. Differences observed in the frequency of CSCs, due to the phenotypic plasticity of many cancer cells, remain a challenge in cancer therapeutics, since CSCs can modulate their transcriptional activities into a more stem-like state to protect themselves from destruction. This plasticity represents an essential step for future therapeutic approaches. Regarding self-renewal, CSCs are modulated by the same molecular pathways found in normal stem cells, such as Wnt/β-catenin signaling, Notch signaling, and Hedgehog signaling. Another key characteristic of CSCs is their resistance to standard chemotherapy and radiotherapy treatments, due to their capacity to rest in a quiescent state. This review will analyze the primary mechanisms involved in CSC tumorigenesis, with particular attention to the roles of CSCs in tumor progression in benign and malignant diseases; and will examine future perspectives on the identification of new markers to better control tumorigenesis, as well as dissecting the metastasis process.
Keywords: cancer; cancer stem cells; drug resistance; metastasis; microenvironment; stem cells; tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Zhu J., Li R., Tiselius E., Roudi R., Teghararian O., Suo C., Song H. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database Syst. Rev. 2017;12:CD011300. doi: 10.1002/14651858.CD011300.pub2. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

