Immunotherapy for Colorectal Cancer: Mechanisms and Predictive Biomarkers
- PMID: 35205776
- PMCID: PMC8869923
- DOI: 10.3390/cancers14041028
Immunotherapy for Colorectal Cancer: Mechanisms and Predictive Biomarkers
Abstract
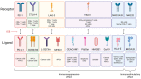
Though early-stage colorectal cancer has a high 5 year survival rate of 65-92% depending on the specific stage, this probability drops to 13% after the cancer metastasizes. Frontline treatments for colorectal cancer such as chemotherapy and radiation often produce dose-limiting toxicities in patients and acquired resistance in cancer cells. Additional targeted treatments are needed to improve patient outcomes and quality of life. Immunotherapy involves treatment with peptides, cells, antibodies, viruses, or small molecules to engage or train the immune system to kill cancer cells. Preclinical and clinical investigations of immunotherapy for treatment of colorectal cancer including immune checkpoint blockade, adoptive cell therapy, monoclonal antibodies, oncolytic viruses, anti-cancer vaccines, and immune system modulators have been promising, but demonstrate limitations for patients with proficient mismatch repair enzymes. In this review, we discuss preclinical and clinical studies investigating immunotherapy for treatment of colorectal cancer and predictive biomarkers for response to these treatments. We also consider open questions including optimal combination treatments to maximize efficacy, minimize toxicity, and prevent acquired resistance and approaches to sensitize mismatch repair-proficient patients to immunotherapy.
Keywords: NK cell; T cell; adoptive cell therapy; anti-cancer vaccines; checkpoint blockade; colorectal cancer; cytokine; immunotherapy; monoclonal antibodies; oncolytic viruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

