Tumor Cell-Autonomous Pro-Metastatic Activities of PD-L1 in Human Breast Cancer Are Mediated by PD-L1-S283 and Chemokine Axes
- PMID: 35205789
- PMCID: PMC8870053
- DOI: 10.3390/cancers14041042
Tumor Cell-Autonomous Pro-Metastatic Activities of PD-L1 in Human Breast Cancer Are Mediated by PD-L1-S283 and Chemokine Axes
Erratum in
-
Correction: Erlichman et al. Tumor Cell-Autonomous Pro-Metastatic Activities of PD-L1 in Human Breast Cancer Are Mediated by PD-L1-S283 and Chemokine Axes. Cancers 2022, 14, 1042.Cancers (Basel). 2022 May 24;14(11):2579. doi: 10.3390/cancers14112579. Cancers (Basel). 2022. PMID: 35681798 Free PMC article.
Abstract
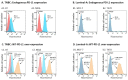
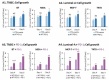
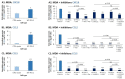
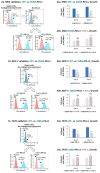
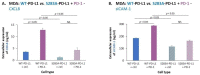
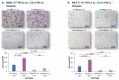
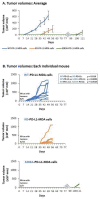
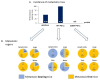
Therapies targeting the PD-L1/PD-1 axis have recently been introduced to triple-negative breast cancer (TNBC) with limited efficacy, suggesting that this axis promotes tumor progression through mechanisms other than immune suppression. Here, we over-expressed WT-PD-L1 in human TNBC cells (express endogenous PD-L1) and in luminal-A breast cancer cells (no endogenous PD-L1 expression) and demonstrated that cell-autonomous PD-L1 activities lead to increased tumor cell growth, invasion and release of pro-metastatic factors (CXCL8, sICAM-1, GM-CSF). These activities were promoted by PD-1 and were inhibited by mutating S283 in PD-L1. Invasion of WT-PD-L1-cells required signaling by chemokine receptors CXCR1/2, CCR2 and CCR5 through autocrine circuits involving CXCL8, CCL2 and CCL5. Studies with T cell-deficient mice demonstrated that cell-autonomous WT-PD-L1 activities in TNBC cells increased tumor growth and metastasis compared to knock-out (KO)-PD-L1-cells, whereas S283A-PD-L1-expressing cells had minimal ability to form tumors and did not metastasize. Overall, our findings reveal autonomous and PD-1-induced tumor-promoting activities of PD-L1 that depend on S283 and on chemokine circuits. These results suggest that TNBC patients whose tumors express PD-L1 could benefit from therapies that prevent immune suppression by targeting PD-1/CTLA-4, alongside with antibodies to PD-L1, which would allow maximal impact by mainly targeting the cancer cells.
Keywords: CCR2; CCR5; CXCR1/2; PD-1; PD-L1; chemokines; luminal-A breast cancer; triple-negative breast cancer (TNBC).
Conflict of interest statement
The authors declare that they do not have a financial relationship with the organizations that sponsored the research. The authors also declare that they do not have any non-financial competing interests.
Figures




References
-
- Gerratana L., Fanotto V., Bonotto M., Bolzonello S., Andreetta C., Moroso S., Pascoletti G., Fasole G., Puglisi F. Pattern of metastatic spread and prognosis of breast cancer biologic subtypes. J. Clin. Oncol. 2014;32:e12532. doi: 10.1200/jco.2014.32.15_suppl.e12532. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

