The Feasibility of Implementing Mainstream Germline Genetic Testing in Routine Cancer Care-A Systematic Review
- PMID: 35205807
- PMCID: PMC8870548
- DOI: 10.3390/cancers14041059
The Feasibility of Implementing Mainstream Germline Genetic Testing in Routine Cancer Care-A Systematic Review
Abstract
Background: Non-genetic healthcare professionals can provide pre-test counseling and order germline genetic tests themselves, which is called mainstream genetic testing. In this systematic review, we determined whether mainstream genetic testing was feasible in daily practice while maintaining quality of genetic care.
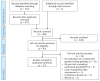
Methods: PubMed, Embase, CINAHL, and PsychINFO were searched for articles describing mainstream genetic testing initiatives in cancer care.
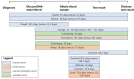
Results: Seventeen articles, reporting on 15 studies, met the inclusion criteria. Non-genetic healthcare professionals concluded that mainstream genetic testing was possible within the timeframe of a routine consultation. In 14 studies, non-genetic healthcare professionals completed some form of training about genetics. When referral was coordinated by a genetics team, the majority of patients carrying a pathogenic variant were seen for post-test counseling by genetic healthcare professionals. The number of days between cancer diagnosis and test result disclosure was always lower in the mainstream genetic testing pathway than in the standard genetic testing pathway (e.g., pre-test counseling at genetics department).
Conclusions: Mainstream genetic testing seems feasible in daily practice with no insurmountable barriers. A structured pathway with a training procedure is desirable, as well as a close collaboration between genetics and other clinical departments.
Keywords: cancer; feasibility; genetic counseling; mainstream genetic testing; quality of care; systematic review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Daly M.B., Pal T., Berry M.P., Buys S.S., Dickson P., Domchek S.M., Elkhanany A., Friedman S., Goggins M., Hutton M.L., et al. NCCN Clinical Practice Guideline in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021;19:77–102. doi: 10.6004/jnccn.2021.0001. - DOI - PubMed
-
- Yadav S., Hu C., Hart S.N., Boddicker N., Polley E.C., Na J., Gnanaolivu R., Lee K.Y., Lindstrom T., Armasu S., et al. Evaluation of Germline Genetic Testing Criteria in a Hospital-Based Series of Women With Breast Cancer. J. Clin. Oncol. 2020;38:1409–1418. doi: 10.1200/JCO.19.02190. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

