Novel Therapeutic Options for Solitary Fibrous Tumor: Antiangiogenic Therapy and Beyond
- PMID: 35205812
- PMCID: PMC8870479
- DOI: 10.3390/cancers14041064
Novel Therapeutic Options for Solitary Fibrous Tumor: Antiangiogenic Therapy and Beyond
Abstract
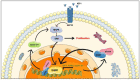
SFT is an ultrarare mesenchymal ubiquitous tumor, with an incidence rate <1 case/million people/year. The fifth WHO classification published in April 2020 subdivided SFT into three categories: benign (locally aggressive), NOS (rarely metastasizing), and malignant. Recurrence can occur in up to 10-40% of localized SFTs, and several risk stratification models have been proposed to predict the individual risk of metastatic relapse. The Demicco model is the most widely used and is based on age at presentation, tumor size, and mitotic count. Total en bloc resection is the standard treatment of patients with a localized SFT; in case of advanced disease, the clinical efficacy of conventional chemotherapy remains poor. In this review, we discuss new insights into the biology and the treatment of patients with SFT. NAB2-STAT6 oncogenic fusion, which is the pathognomonic hallmark of SFT, is supposedly involved in the overexpression of vascular endothelial growth factor (VEGF). These specific biological features encouraged the successful assessment of antiangiogenic drugs. Overall, antiangiogenic therapies showed a significant activity toward SFT in the advanced/metastatic setting. Nevertheless, these promising results warrant additional investigation to be validated, including randomized phase III trials and biological translational analysis, to understand and predict mechanisms of efficacy and resistance. While the therapeutic potential of immunotherapy remains elusive, the use of antiangiogenics as first-line treatment should be considered.
Keywords: SFT; antiangiogenics; immune-checkpoint inhibitors; rare sarcoma.
Conflict of interest statement
J.-Y. Blay reports grants and personal fees from PharmaMar and AstraZeneca outside the submitted work. I. Ray-Coquard reports personal fees from Roche, PharmaMar, AstraZeneca, Clovis, GlaxoSmithKline, Bristol Myers Squibb, Agenus, Mersana, ImmunoGen, MSD, EISAI, and Novartis outside the submitted work. The other authors declare no conflict of interest.
Figures


References
-
- Diebold M., Soltermann A., Hottinger S., Haile S., Bubendorf L., Komminoth P., Jochum W., Grobholz R., Theegarten D., Berezowska S., et al. Prognostic value of MIB-1 proliferation index in solitary fibrous tumors of the pleura implemented in a new score—Amulticenter study. Respir. Res. 2017;18:210. doi: 10.1186/s12931-017-0693-8. - DOI - PMC - PubMed
-
- Pasquali S., Gronchi A., Strauss D., Bonvalot S., Jeys L., Stacchiotti S., Hayes A., Honore C., Collini P., Renne S., et al. Resectable extra-pleural and extra-meningeal solitary fibrous tumours: A multi-centre prognostic study. Eur. J. Surg. Oncol. (EJSO) 2016;42:1064–1070. doi: 10.1016/j.ejso.2016.01.023. - DOI - PubMed
-
- Salas S., Resseguier N., Blay J.Y., Le Cesne A., Italiano A., Chevreau C., Rosset P., Isambert N., Soulie P., Cupissol D., et al. Prediction of local and metastatic recurrence in solitary fibrous tumor: Construction of a risk calculator in a multicenter cohort from the French Sarcoma Group (FSG) database. Ann. Oncol. 2017;28:1779–1787. doi: 10.1093/annonc/mdx250. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

