COVID-19 Associated Pulmonary Aspergillosis: Diagnostic Performance, Fungal Epidemiology and Antifungal Susceptibility
- PMID: 35205848
- PMCID: PMC8875712
- DOI: 10.3390/jof8020093
COVID-19 Associated Pulmonary Aspergillosis: Diagnostic Performance, Fungal Epidemiology and Antifungal Susceptibility
Abstract
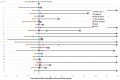
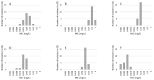
Coronavirus disease 2019 (COVID-19)-associated pulmonary aspergillosis (CAPA) raises concerns as to whether it contributes to an increased mortality. The incidence of CAPA varies widely within hospitals and countries, partly because of difficulties in obtaining a reliable diagnosis. We implemented a routine screening of respiratory specimens in COVID-19 ICU patients for Aspergillus species using culture and galactomannan (GM) detection from serum and/or bronchoalveolar lavages (BAL). Out of 329 ICU patients treated during March 2020 and April 2021, 23 (7%) suffered from CAPA, 13 of probable, and 10 of possible. In the majority of cases, culture, microscopy, and GM testing were in accordance with CAPA definition. However, we saw that the current definitions underscore to pay attention for fungal microscopy and GM detection in BALs, categorizing definitive CAPA diagnosis based on culture positive samples only. The spectrum of Aspergillus species involved Aspergillus fumigatus, followed by Aspergillus flavus, Aspergillus niger, and Aspergillus nidulans. We noticed changes in fungal epidemiology, but antifungal resistance was not an issue in our cohort. The study highlights that the diagnosis and incidence of CAPA is influenced by the application of laboratory-based diagnostic tests. Culture positivity as a single microbiological marker for probable definitions may overestimate CAPA cases and thus may trigger unnecessary antifungal treatment.
Keywords: COVID-19; antifungal susceptibility testing; aspergillosis; coronavirus disease 2019 (COVID-19)-associated pulmonary aspergillosis (CAPA); fungal diagnosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ullmann A.J., Aguado J.M., Arikan-Akdagli S., Denning D.W., Groll A.H., Lagrou K., Lass-Flörl C., Lewis R.E., Munoz P., Verweij P.E., et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018;24:E1–E38. doi: 10.1016/j.cmi.2018.01.002. - DOI - PubMed
-
- Verweij P.E., Rijnders B.J.A., Bruggemann R.J.M., Azoulay E., Bassetti M., Blot S., Calandra T., Clancy C.J., Cornely O.A., Chiller T., et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: An expert opinion. Intens. Care Med. 2020;46:1524–1535. doi: 10.1007/s00134-020-06091-6. - DOI - PMC - PubMed
-
- Schauwvlieghe A., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C., Lagrou K., Verweij P.E., Van de Veerdonk F.L., Gommers D., et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. - DOI - PubMed
-
- Koehler P., Bassetti M., Chakrabarti A., Chen S.C.A., Colombo A.L., Hoenigl M., Klimko N., Lass-Florl C., Oladele R.O., Vinh D.C., et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021;21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

