Identification of β-Glucosidase Activity of Lentilactobacillus buchneri URN103L and Its Potential to Convert Ginsenoside Rb1 from Panax ginseng
- PMID: 35206006
- PMCID: PMC8870947
- DOI: 10.3390/foods11040529
Identification of β-Glucosidase Activity of Lentilactobacillus buchneri URN103L and Its Potential to Convert Ginsenoside Rb1 from Panax ginseng
Abstract
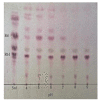
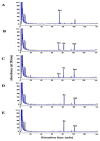
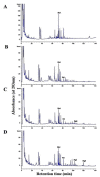
Lentilactobacillus buchneri isolated from Korean fermented plant foods produces β-glucosidase, which can hydrolyze ginsenoside Rb1 from Panax ginseng to yield ginsenoside Rd. The aim of this study was to determine the mechanisms underlying the extracellular β-glucosidase activity obtained from Lentilactobacillus buchneri URN103L. Among the 17 types of lactic acid bacteria showing positive β-glucosidase activity in the esculin iron agar test, only URN103L was found to exhibit high hydrolytic activity on ginsenoside Rb1. The strain showed 99% homology with Lentilactobacillus buchneri NRRLB 30929, whereby it was named Lentilactobacillus buchneri URN103L. Supernatants of selected cultures with β-glucosidase activity were examined for hydrolysis of the major ginsenoside Rb1 at 40 °C, pH 5.0. Furthermore, the β-glucosidase activity of this strain showed a distinct ability to hydrolyze major ginsenoside Rb1 into minor ginsenosides Rd and Rg3. Lentilactobacillus buchneri URN103L showed higher leucine arylamidase, valine arylamidase, α-galactosidass, β-galactosidase, and β-glucosidase activities than any other strain. We conclude that β-glucosidase from Lentilactobacillus buchneri URN103L can effectively hydrolyze ginsenoside Rb1 into Rd and Rg3. The converted ginsenoside can be used in functional foods, yogurts, beverage products, cosmetics, and other health products.
Keywords: Lentilactobacillus buchneri URN103L; Rd; Rg3; ginsenoside Rb1; hydrolyze; β-glucosidase.
Conflict of interest statement
The authors declare no conflict of interests.
Figures






References
-
- Kim M.W., Ko S.R., Choi K.J., Kim S.C. Distribution of saponin in various sections of Panax ginseng root and changes of its content according to root age. J. Ginseng Res. 1987;11:10–16.
-
- Jia L., Zhao Y., Liang X.J. Current evaluation of the millennium phytomedicine- ginseng (II): Collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr. Med. Chem. 2009;16:2924–2942. doi: 10.2174/092986709788803204. - DOI - PMC - PubMed
-
- Park J.S., Shin J.A., Jung J.S., Hyun J.W., Van Le T.K., Kim D.H., Park E.M., Kim H.S. Anti-inflammatory mechanism of compound K in activated microglia and its neuroprotective effect on experimental stroke in mice. J. Pharmacol. Exp. Ther. 2012;341:59–67. doi: 10.1124/jpet.111.189035. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

