Clinical Comparison of a Novel Balloon-Expandable Versus a Self-Expanding Transcatheter Heart Valve for the Treatment of Patients with Severe Aortic Valve Stenosis: The EVAL Registry
- PMID: 35207232
- PMCID: PMC8876233
- DOI: 10.3390/jcm11040959
Clinical Comparison of a Novel Balloon-Expandable Versus a Self-Expanding Transcatheter Heart Valve for the Treatment of Patients with Severe Aortic Valve Stenosis: The EVAL Registry
Abstract
Background: Transcatheter aortic valve replacement (TAVR) is an effective treatment option for patients with severe, symptomatic AS, regardless of the transcatheter heart valve (THV) implanted. Prior studies demonstrated a higher device success with lower paravalvular leak (PVL) using the balloon-expandable (BE) Sapien/XT THV vs. a self-expanding (SE) THV. However, few data are available on the performance of a novel BE THV.
Purpose: to compare early clinical performance and safety of the newly available BE Myval THV (Myval, Meril Life Sciences Pvt. Ltd., India) vs. the commonly used SE (Evolut R, Medtronic) THV.
Methods: A single-center, retrospective cohort analysis was performed with 166 consecutive patients undergoing TAVR from March 2019 to March 2021 for severe symptomatic AS treated with either the novel BE Myval or the SE Evolut R (ER) bioprosthesis. The primary endpoint was device success at day 30 according to the Valve Academic Research Consortium-3 (VARC-3). Secondary endpoints included 30-day all-cause mortality, cardiovascular mortality, more than mild PVL, permanent pacemaker implantation (PPI) rates and a composite of all-cause mortality and disabling stroke at 6 months.
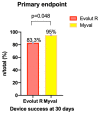
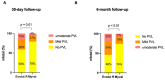
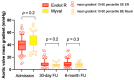
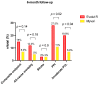
Results: Among the 166 included patients, 108 patients received the SE ER THV and 58 patients were treated with the BE Myval THV. At baseline, the two groups showed comparable demographic characteristics. The primary composite endpoint of early device success occurred in 55 patients (94.8%) in the BE Myval group and in 90 patients (83.3%) in the SE ER group (OR 3.667, 95% CI 1.094-12.14; p = 0.048). At day 30, the BE Myval THV group exhibited a significantly lower incidence of more than mild PVL (BE Myval 3.45% vs. SE ER 14.8%, OR 0.2, 95% CI 0.05-0.8; p = 0.0338), along with a lower rate of PPI (BE Myval 11% vs. SE ER 24.2%, OR 0.38, 95% CI 0.15-0.99; p = 0.0535). At the 6-month follow-up, the incidence of all-cause mortality and disabling stroke did not significantly differ between the two groups, while the incidence of PPI (BE Myval 11% vs. SE ER 27.5%, OR 0.32, CI 95% 0.1273-0.8; p = 0.02) and ≥moderate PVL (BE Myval 6.9% vs. SE ER 19.8%, OR 0.31, 95% CI 0.1-0.94; p = 0.0396) was significantly lower in the BE Myval group.
Conclusions: In patients with severe symptomatic AS undergoing TAVR, the novel Myval BE THV provided a comparable performance to the well-known ER SE THV, and it was associated with a lower rate of PPI and ≥moderate PVL within 30 days and 6 months after the procedure. Randomized, head-to-head comparison trials are needed to confirm our results.
Keywords: balloon-expandable Myval (BE Myval); paravalvular leak (PVL); permanent pacemaker implantation (PPI); self-expanding CoreValve Evolut R (SE ER); transcatheter aortic valve replacement (TAVR).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cribier A., Eltchaninoff H., Bash A., Borenstein N., Tron C., Bauer F., Derumeaux G., Anselme F., Laborde F., Leon M.B. Percutaneous Transcatheter Implantation of an Aortic Valve Prosthesis for Calcific Aortic Stenosis. Circulation. 2002;106:3006–3008. doi: 10.1161/01.CIR.0000047200.36165.B8. - DOI - PubMed
-
- Leon M.B., Smith C.R., Mack M., Miller D.C., Moses J.W., Svensson L.G., Tuzcu E.M., Webb J.G., Fontana G.P., Makkar R.R., et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med. 2010;17:1597–1607. doi: 10.1056/NEJMoa1008232. - DOI - PubMed
-
- Adams D.H., Popma J.J., Reardon M.J., Yakubov S.J., Coselli J.S., Deeb G.M., Gleason T.G., Buchbinder M., Hermiller J., Kleiman N.S., et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis Abstract. N. Engl. J. Med. 2014;19:1790–1798. doi: 10.1056/NEJMoa1400590. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

