Persistence of Cryoglobulinemic Vasculitis after DAA Induced HCV Cure
- PMID: 35207257
- PMCID: PMC8878349
- DOI: 10.3390/jcm11040984
Persistence of Cryoglobulinemic Vasculitis after DAA Induced HCV Cure
Abstract
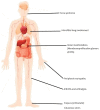
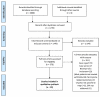
Treatment with a direct acting antiviral (DAA) has revolutionized HCV therapy, as more than 95% of patients achieve a sustained virological response (SVR). Cryoglobulinemic vasculitis (CryoVas), however, can persist and recur after the HCV cure. In this systematic review, we include data from 19 studies that provided information on the persistence and recurrence of CryoVas after the HCV cure with DAAs. A complete clinical response (CR) was reported in 63.7% to 90.2% of the DAA-treated patients after achieving SVR. Relapse of CryoVas symptoms was reported in 4% to 18% of the patients. Neuropathy, nephropathy, and dermatological complications were the most common manifestations of CryoVas. B-cell clones persisted in 31-40% of the patients and could contribute to CryoVas relapse. INFL3-rs12979860, ARNTL-rs648122, RETN-rs1423096, and SERPINE1-rs6976053 were associated with a higher incidence of persistence and recurrence of CryoVas. Prospective multicenter studies with diverse patient populations are needed to validate these findings for the timely and effective management of this challenging condition.
Keywords: DAA therapy; cryoglobulinemia; cryoglobulinemic vasculitis; hepatitis C.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Relapse of Hepatitis C Virus Cryoglobulinemic Vasculitis After Sustained Viral Response After Interferon-Free Direct-Acting Antivirals.Am J Gastroenterol. 2022 Apr 1;117(4):627-636. doi: 10.14309/ajg.0000000000001667. Am J Gastroenterol. 2022. PMID: 35103020
-
Long-term Efficacy of Interferon-Free Antiviral Treatment Regimens in Patients With Hepatitis C Virus-Associated Cryoglobulinemia Vasculitis.Clin Gastroenterol Hepatol. 2019 Feb;17(3):518-526. doi: 10.1016/j.cgh.2018.05.021. Epub 2018 May 29. Clin Gastroenterol Hepatol. 2019. PMID: 29857143
-
Effectiveness and cost of hepatitis C virus cryoglobulinaemia vasculitis treatment: From interferon-based to direct-acting antivirals era.Liver Int. 2017 Dec;37(12):1805-1813. doi: 10.1111/liv.13465. Epub 2017 May 29. Liver Int. 2017. PMID: 28467688
-
Hepatitis C virus-related vasculitis.Clin Res Hepatol Gastroenterol. 2021 Sep;45(5):101575. doi: 10.1016/j.clinre.2020.11.005. Epub 2020 Nov 29. Clin Res Hepatol Gastroenterol. 2021. PMID: 33268038 Review.
-
The evolving scenario of HCV-related mixed cryoglobulinemia and B-cell lymphoma in the era of direct-acting antivirals.Expert Rev Anti Infect Ther. 2025 Jan;23(1):19-30. doi: 10.1080/14787210.2024.2442475. Epub 2025 Jan 3. Expert Rev Anti Infect Ther. 2025. PMID: 39749733 Review.
Cited by
-
Renal Manifestations of Chronic Hepatitis C: A Review.J Clin Med. 2024 Sep 18;13(18):5536. doi: 10.3390/jcm13185536. J Clin Med. 2024. PMID: 39337023 Free PMC article. Review.
-
Chronic Hepatitis C Virus Infection, Extrahepatic Disease and the Impact of New Direct-Acting Antivirals.Pathogens. 2024 Apr 19;13(4):339. doi: 10.3390/pathogens13040339. Pathogens. 2024. PMID: 38668294 Free PMC article. Review.
-
Therapeutic Potential of Rituximab in Managing Hepatitis C-Associated Cryoglobulinemic Vasculitis: A Systematic Review.J Clin Med. 2023 Oct 27;12(21):6806. doi: 10.3390/jcm12216806. J Clin Med. 2023. PMID: 37959271 Free PMC article. Review.
-
Hepatitis C Virus-associated Cryoglobulinemic Livedo Reticularis Improved with Direct-acting Antivirals.Intern Med. 2023 Dec 15;62(24):3631-3636. doi: 10.2169/internalmedicine.1671-23. Epub 2023 Apr 28. Intern Med. 2023. PMID: 37121750 Free PMC article.
-
Virus-Associated Nephropathies: A Narrative Review.Int J Mol Sci. 2022 Oct 10;23(19):12014. doi: 10.3390/ijms231912014. Int J Mol Sci. 2022. PMID: 36233315 Free PMC article. Review.
References
-
- Retamozo S., Brito-Zerón P., Bosch X., Stone J.H., Ramos-Casals M. Cryoglobulinemic disease. Oncology. 2013;27:1098–1116. - PubMed
-
- Santer D.M., Ma M.M., Hockman D., Landi A., Tyrrell D.L., Houghton M. Enhanced activation of memory, but not naïve, B cells in chronic hepatitis C virus-infected patients with cryoglobulinemia and advanced liver fibrosis. PLoS ONE. 2013;8:e68308. doi: 10.1371/journal.pone.0068308. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous