Switching from Adalimumab Originator to Biosimilar: Clinical Experience in Patients with Hidradenitis Suppurativa
- PMID: 35207280
- PMCID: PMC8879480
- DOI: 10.3390/jcm11041007
Switching from Adalimumab Originator to Biosimilar: Clinical Experience in Patients with Hidradenitis Suppurativa
Abstract
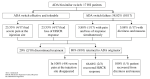
Adalimumab is currently the only biological medicine approved by the FDA for the treatment of hidradenitis suppurativa (HS). The breakout of biosimilar drugs made them more accessible due to their impact on pharmacoeconomics. However, packaging, formulation, or excipients are unique characteristics of each drug. In that way, switching from adalimumab originator to biosimilar and between biosimilars could have implications in the clinical practice. The objective of this study is to describe our clinical experience in switching from adalimumab originator to biosimilar and switching back again. A single-center retrospective cohort study was conducted that included seventeen patients with HS treated with adalimumab originator in the maintenance phase, and that achieved Hidradenitis Suppurativa Clinical Response (HiSCR), who were switched to adalimumab biosimilar for no medical reasons. The reason for the change was to improve pharmacoeconomic efficiency, following our hospital policies on biologics. Median duration with adalimumab originator treatment before switching was 48 weeks. After switching, 41.2% of patients maintained HiSCR response without additional issues, while 58.8% (10/17) reported problems after the change. Switching from adalimumab originator to biosimilar in well-controlled patients could imply problems in efficacy and adherence. Switching back to adalimumab originator appears to solve most of the problems, but some patients can lose confidence in the drug and discontinue it. It would be worthwhile to evaluate the benefit-risk ratio individually when switching an HS patient to adalimumab biosimilar.
Keywords: adalimumab; biosimilar; hidradenitis suppurativa; switching.
Conflict of interest statement
The authors declare no conflict of interest.
References
-
- Kimball A., Sobell J., Zouboulis C.P.D., Gu Y., Williams D.A., Sundaram M., Teixeira H., Jemec G. HiSCR (Hidradenitis Suppurativa Clinical Response): A novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J. Eur. Acad. Dermatol. Venereol. 2015;30:989–994. doi: 10.1111/jdv.13216. - DOI - PMC - PubMed
-
- US Food and Drug Administration HUMIRA® (adalimumab) Injection, for Subcutaneous Use. [(accessed on 8 April 2008)];2008 Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/125057s0110lbl....
-
- Puig L., Lopez-Ferrer A., Vilarrasa E., Garcia I., Fernandez-del Olmo R. Model for assessing the efficiency of biologic drugs in the treatment of moderate to severe psoriasis for one year in clinical practice in Spain. Actas Dermosifiliogr. 2016;107:34–43. doi: 10.1016/j.ad.2015.07.006. - DOI - PubMed