Elexacaftor/Tezacaftor/Ivacaftor in Patients with Cystic Fibrosis Homozygous for the F508del Mutation and Advanced Lung Disease: A 48-Week Observational Study
- PMID: 35207295
- PMCID: PMC8876133
- DOI: 10.3390/jcm11041021
Elexacaftor/Tezacaftor/Ivacaftor in Patients with Cystic Fibrosis Homozygous for the F508del Mutation and Advanced Lung Disease: A 48-Week Observational Study
Abstract
Background: Elexacaftor/tezacaftor/ivacaftor (ETI) is the newest cystic fibrosis transmembrane conductance regulator (CFTR) modulator drug approved for the treatment of patients with cystic fibrosis (pwCF) aged ≥6 years with at least one copy of the F508del mutation (F) in the CFTR gene or another mutation that is responsive to treatment with ETI. This study determined the effectiveness and safety of ETI in a cohort of severely affected pwCF with an F/F genotype.
Methods: Retrospective observational study in F/F pwCF treated for 48 weeks, enrolled in an ETI managed access program available to subjects with advanced lung disease (ppFEV1 < 40). Twenty-six patients from three centres were included. The main outcomes included lung function, sweat chloride concentration (SCC), nutrition, frequency of pulmonary exacerbations (PEx), CFQ-R, and safety.
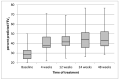
Results: ppFEV1 improved by 12.06 (95%CI 8.54, 15.57) from baseline after 4 weeks of treatment with ETI, 15.32 (11.3, 19.34) after 24 weeks, and 14.48 (10.64, 18.32) after 48 weeks. The increase in FEV1 was accompanied by a decrease in SCC, improvement of BMI, and noticeable reduction in PEx. An overall good safety profile was observed.
Conclusions: In F/F pwCF with advanced lung disease with an F/F genotype, ETI was safe and associated with clinical improvement.
Keywords: advanced lung disease; cystic fibrosis; elexacaftor/tezacaftor/ivacaftor.
Conflict of interest statement
D.S. has been principal investigator in several clinical trials conducted by Vertex Pharmaceuticals and for which his institution has received funding; he has received fees for participation in advisory boards, satellite symposia, or specific projects. V.C. and PI have received fees for participation in advisory boards and consultancy from Vertex Pharmaceuticals. C.C., G.M. and M.D. report supported attendance at meetings and travel from Vertex Pharmaceuticals. V.T., L.F., M.F., A.P., S.B., A.C., L.S. and N.F. have nothing to disclose.
Figures
Similar articles
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2020 Dec 17;12(12):CD010966. doi: 10.1002/14651858.CD010966.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Nov 20;11:CD010966. doi: 10.1002/14651858.CD010966.pub4. PMID: 33331662 Free PMC article. Updated.
-
Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: a 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial.Lancet Respir Med. 2022 Mar;10(3):267-277. doi: 10.1016/S2213-2600(21)00454-9. Epub 2021 Dec 20. Lancet Respir Med. 2022. PMID: 34942085 Clinical Trial.
-
Safety and efficacy of vanzacaftor-tezacaftor-deutivacaftor in adults with cystic fibrosis: randomised, double-blind, controlled, phase 2 trials.Lancet Respir Med. 2023 Jun;11(6):550-562. doi: 10.1016/S2213-2600(22)00504-5. Epub 2023 Feb 23. Lancet Respir Med. 2023. PMID: 36842446 Clinical Trial.
-
Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial.Lancet. 2019 Nov 23;394(10212):1940-1948. doi: 10.1016/S0140-6736(19)32597-8. Epub 2019 Oct 31. Lancet. 2019. PMID: 31679946 Free PMC article. Clinical Trial.
-
Correctors (specific therapies for class II CFTR mutations) for cystic fibrosis.Cochrane Database Syst Rev. 2018 Aug 2;8(8):CD010966. doi: 10.1002/14651858.CD010966.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 Dec 17;12:CD010966. doi: 10.1002/14651858.CD010966.pub3. PMID: 30070364 Free PMC article. Updated. Review.
Cited by
-
Real-life efficacy and safety of elexacaftor/tezacaftor/ivacaftor on severe cystic fibrosis lung disease patients.Pharmacol Res Perspect. 2022 Dec;10(6):e01015. doi: 10.1002/prp2.1015. Pharmacol Res Perspect. 2022. PMID: 36440690 Free PMC article.
-
Is Obesity a Problem in New Cystic Fibrosis Treatments?Nutrients. 2024 Sep 14;16(18):3103. doi: 10.3390/nu16183103. Nutrients. 2024. PMID: 39339703 Free PMC article.
-
Efficacy and Safety of Elexacaftor-Tezacaftor-Ivacaftor in the Treatment of Cystic Fibrosis: A Systematic Review.Children (Basel). 2023 Mar 15;10(3):554. doi: 10.3390/children10030554. Children (Basel). 2023. PMID: 36980112 Free PMC article. Review.
-
One year of treatment with elexacaftor/tezacaftor/ivacaftor in patients with cystic fibrosis homozygous for the F508del mutation causes a significant increase in liver biochemical indexes.Front Mol Biosci. 2024 Jan 8;10:1327958. doi: 10.3389/fmolb.2023.1327958. eCollection 2023. Front Mol Biosci. 2024. PMID: 38259684 Free PMC article.
-
Longitudinal Study of Therapeutic Adherence in a Cystic Fibrosis Unit: Identifying Potential Factors Associated with Medication Possession Ratio.Antibiotics (Basel). 2022 Nov 16;11(11):1637. doi: 10.3390/antibiotics11111637. Antibiotics (Basel). 2022. PMID: 36421281 Free PMC article.
References
-
- Konstan M.W., McKone E.F., Moss R.B., Marigowda G., Tian S., Waltz D., Huang X., Lubarsky B., Rubin J., Millar S.J., et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): A phase 3, extension study. Lancet Respir. Med. 2017;5:107–118. doi: 10.1016/S2213-2600(16)30427-1. - DOI - PubMed
-
- Wainwright C.E., Elborn J.S., Ramsey B.W., Marigowda G., Huang X., Cipolli M., Colombo C., Davies J.C., de Boeck K., Flume P.A., et al. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for F508del CFTR. N. Engl. J. Med. 2015;373:220–231. doi: 10.1056/NEJMoa1409547. - DOI - PMC - PubMed
-
- Taylor-Cousar J.L., Jain M., Barto T.L., Haddad T., Atkinson J., Tian S., Tang R., Marigowda G., Waltz D., Pilewski J., et al. Lumacaftor/ivacaftor in patients with cystic fibrosis and advanced lung disease homozygous for F508del-CFTR. J. Cyst. Fibros. 2018;17:228–235. doi: 10.1016/j.jcf.2017.09.012. - DOI - PubMed
-
- McColley S.A., Konstan M.W., Ramsey B.W., Stuart Elborn J., Boyle M.P., Wainwright C.E., Waltz D., Vera Llonch M., Marigowda G., Jiang J.G., et al. Lumacaftor/Ivacaftor reduces pulmonary exacerbations in patients irrespective of initial changes in FEV1. J. Cyst. Fibros. 2019;18:94–101. doi: 10.1016/j.jcf.2018.07.011. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

