Elexacaftor/Tezacaftor/Ivacaftor in Patients with Cystic Fibrosis Homozygous for the F508del Mutation and Advanced Lung Disease: A 48-Week Observational Study
- PMID: 35207295
- PMCID: PMC8876133
- DOI: 10.3390/jcm11041021
Elexacaftor/Tezacaftor/Ivacaftor in Patients with Cystic Fibrosis Homozygous for the F508del Mutation and Advanced Lung Disease: A 48-Week Observational Study
Abstract
Background: Elexacaftor/tezacaftor/ivacaftor (ETI) is the newest cystic fibrosis transmembrane conductance regulator (CFTR) modulator drug approved for the treatment of patients with cystic fibrosis (pwCF) aged ≥6 years with at least one copy of the F508del mutation (F) in the CFTR gene or another mutation that is responsive to treatment with ETI. This study determined the effectiveness and safety of ETI in a cohort of severely affected pwCF with an F/F genotype.
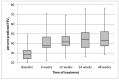
Methods: Retrospective observational study in F/F pwCF treated for 48 weeks, enrolled in an ETI managed access program available to subjects with advanced lung disease (ppFEV1 < 40). Twenty-six patients from three centres were included. The main outcomes included lung function, sweat chloride concentration (SCC), nutrition, frequency of pulmonary exacerbations (PEx), CFQ-R, and safety.
Results: ppFEV1 improved by 12.06 (95%CI 8.54, 15.57) from baseline after 4 weeks of treatment with ETI, 15.32 (11.3, 19.34) after 24 weeks, and 14.48 (10.64, 18.32) after 48 weeks. The increase in FEV1 was accompanied by a decrease in SCC, improvement of BMI, and noticeable reduction in PEx. An overall good safety profile was observed.
Conclusions: In F/F pwCF with advanced lung disease with an F/F genotype, ETI was safe and associated with clinical improvement.
Keywords: advanced lung disease; cystic fibrosis; elexacaftor/tezacaftor/ivacaftor.
Conflict of interest statement
D.S. has been principal investigator in several clinical trials conducted by Vertex Pharmaceuticals and for which his institution has received funding; he has received fees for participation in advisory boards, satellite symposia, or specific projects. V.C. and PI have received fees for participation in advisory boards and consultancy from Vertex Pharmaceuticals. C.C., G.M. and M.D. report supported attendance at meetings and travel from Vertex Pharmaceuticals. V.T., L.F., M.F., A.P., S.B., A.C., L.S. and N.F. have nothing to disclose.
Figures
References
-
- Konstan M.W., McKone E.F., Moss R.B., Marigowda G., Tian S., Waltz D., Huang X., Lubarsky B., Rubin J., Millar S.J., et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): A phase 3, extension study. Lancet Respir. Med. 2017;5:107–118. doi: 10.1016/S2213-2600(16)30427-1. - DOI - PubMed
-
- Wainwright C.E., Elborn J.S., Ramsey B.W., Marigowda G., Huang X., Cipolli M., Colombo C., Davies J.C., de Boeck K., Flume P.A., et al. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for F508del CFTR. N. Engl. J. Med. 2015;373:220–231. doi: 10.1056/NEJMoa1409547. - DOI - PMC - PubMed
-
- Taylor-Cousar J.L., Jain M., Barto T.L., Haddad T., Atkinson J., Tian S., Tang R., Marigowda G., Waltz D., Pilewski J., et al. Lumacaftor/ivacaftor in patients with cystic fibrosis and advanced lung disease homozygous for F508del-CFTR. J. Cyst. Fibros. 2018;17:228–235. doi: 10.1016/j.jcf.2017.09.012. - DOI - PubMed
-
- McColley S.A., Konstan M.W., Ramsey B.W., Stuart Elborn J., Boyle M.P., Wainwright C.E., Waltz D., Vera Llonch M., Marigowda G., Jiang J.G., et al. Lumacaftor/Ivacaftor reduces pulmonary exacerbations in patients irrespective of initial changes in FEV1. J. Cyst. Fibros. 2019;18:94–101. doi: 10.1016/j.jcf.2018.07.011. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

