IDELVION: A Comprehensive Review of Clinical Trial and Real-World Data
- PMID: 35207344
- PMCID: PMC8875492
- DOI: 10.3390/jcm11041071
IDELVION: A Comprehensive Review of Clinical Trial and Real-World Data
Abstract
Hemophilia B is a bleeding disorder caused by a deficiency of coagulation factor IX (FIX). Treatment with FIX replacement products can increase FIX activity levels to minimize or prevent bleeding events. However, frequent dosing with standard-acting FIX products can create a high treatment burden. Long-acting products have been developed to maintain bleed protection with extended dosing intervals. Recombinant factor IX-albumin fusion protein (rIX-FP) is a long-acting product indicated for the treatment and prophylaxis of bleeding events and perioperative management in adult and pediatric patients. This review outlines data from all previously treated patients in the Prophylaxis and On-Demand Treatment using Longer Half-Life rIX-FP (PROLONG-9FP) clinical trial program and summarizes real-world data evaluating the use of rIX-FP in routine clinical practice. In the PROLONG-9FP program, rIX-FP demonstrated effective hemostasis in all patients at dose regimens of up to 21 days in patients aged ≥ 18 years and up to 14 days in patients aged < 12 years. rIX-FP has a favorable pharmacokinetic profile and an excellent safety and tolerability profile. Extended dosing intervals with rIX-FP led to high levels of adherence and reduced consumption compared with other FIX therapies. Data from real-world practice are encouraging and reflect the results of the clinical trials.
Keywords: albumin fusion protein; annualized bleeding rates; extended half-life; factor IX; hemophilia B.
Conflict of interest statement
M.E. reports consulting honoraria for participation in advisory boards and/or consulting for CSL Behring, Genentech, Biomarin, Kedrion, the National Hemophilia Foundation, Novo Nordisk, Pfizer, Roche, Sanofi, Shire, and Takeda; and research funding from Pfizer, NovoNordisk, Takeda, Sanofi, and UniQure. M.E.M. reports consulting honoraria for participation in advisory boards and/or speaker bureaus for Bayer, Biotest, Biomarin, Catalyst, CSL Behring, Grifols, Kedrion, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, Spark Therapeutics, and Takeda. C.H. reports consulting honoraria for participation in advisory boards and/or speaker bureaus for Bayer, Biogen, CAF-DCF, CSL Behring, Kedrion, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Shire, and SOBI; research funding from Bayer, Shire, and Pfizer; and is a past president of the European Association of Haemophilia and Allied Diseases (EAHAD) and a member of the Board of Directors of the World Federation of Hemophilia (WFH). C.L. reports consulting honoraria for participation in advisory boards for Bayer, Catalyst, CSL Behring, Genentech/Roche, HEMA Biologics, Sanofi, and Takeda. W.S., Y.L., and W.M. are employees of CSL Behring. J.O. has received reimbursement for attending symposia/congresses, honoraria for speaking and/or consulting, and funds for research from Bayer, Biotest, Chugai, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Shire, and Swedish Orphan Biovitrum.
Figures

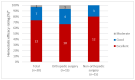
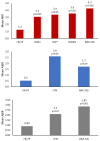
References
-
- Makris M., Oldenburg J., Mauser-Bunschoten E.P., Peerlinck K., Castaman G., Fijnvandraat K., The Subcommittee on Factor VIII, Factor IX and Rare Bleeding DIsorders The definition, diagnosis and management of mild hemophilia A: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2018;16:2530–2533. doi: 10.1111/jth.14315. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

