Cell Cycle Stage and DNA Repair Pathway Influence CRISPR/Cas9 Gene Editing Efficiency in Porcine Embryos
- PMID: 35207459
- PMCID: PMC8876063
- DOI: 10.3390/life12020171
Cell Cycle Stage and DNA Repair Pathway Influence CRISPR/Cas9 Gene Editing Efficiency in Porcine Embryos
Abstract
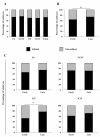
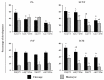
CRISPR/Cas9 technology is a powerful tool used for genome manipulation in different cell types and species. However, as with all new technologies, it still requires improvements. Different factors can affect CRISPR/Cas efficiency in zygotes, which influence the total cost and complexity for creating large-animal models for research. This study evaluated the importance of zygote cell cycle stage between early-injection (within 6 h post activation/fertilization) versus late-injection (14-16 h post activation/fertilization) when the CRISPR/Cas9 components were injected and the inhibition of the homologous recombination (HR) pathway of DNA repair on gene editing, embryo survival and development on embryos produced by fertilization, sperm injection, somatic cell nuclear transfer, and parthenogenetic activation technologies. Injections at the late cell cycle stage decreased embryo survival (measured as the proportion of unlysed embryos) and blastocyst formation (68.2%; 19.3%) compared to early-stage injection (86.3%; 28.8%). However, gene editing was higher in blastocysts from late-(73.8%) vs. early-(63.8%) injected zygotes. Inhibition of the HR repair pathway increased gene editing efficiency by 15.6% in blastocysts from early-injected zygotes without compromising embryo development. Our finding shows that injection at the early cell cycle stage along with HR inhibition improves both zygote viability and gene editing rate in pig blastocysts.
Keywords: DNA repair; cell cycle; gene editing; pigs; zygotes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hauschild J., Petersen B., Santiago Y., Queisser A.L., Carnwath J.W., Lucas-Hahn A., Zhang L., Meng X., Gregory P.D., Schwinzer R., et al. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proc. Natl. Acad. Sci. USA. 2011;108:12013–12017. doi: 10.1073/pnas.1106422108. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

