Oxidative Phosphorus Chemistry Perturbed by Minerals
- PMID: 35207486
- PMCID: PMC8878404
- DOI: 10.3390/life12020198
Oxidative Phosphorus Chemistry Perturbed by Minerals
Abstract
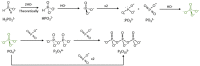
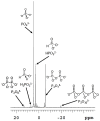
Life is a complex, open chemical system that must be supported with energy inputs. If one fathoms how simple early life must have been, the complexity of modern-day life is staggering by comparison. A minimally complex system that could plausibly provide pyrophosphates for early life could be the oxidation of reduced phosphorus sources such as hypophosphite and phosphite. Like all plausible prebiotic chemistries, this system would have been altered by minerals and rocks in close contact with the evolving solutions. This study addresses the different types of perturbations that minerals might have on this chemical system. This study finds that minerals may inhibit the total production of oxidized phosphorus from reduced phosphorus species, they may facilitate the production of phosphate, or they may facilitate the production of pyrophosphate. This study concludes with the idea that mineral perturbations from the environment increase the chemical complexity of this system.
Keywords: Fenton chemistry; chemical complexity; chemical evolution; minerals; olivine; pyrophosphate; reduced phosphorus; schreibersite; serpentinite; ulexite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Mikhailov A., Ertl G. The Frontiers Collection. Springer; Berlin/Heidelberg, Germany: 2017. Chemical complexity.
-
- Pasek M.A., Gull M., Herschy B. Phosphorylation on the early earth. Chem. Geol. 2017;475:149–170. doi: 10.1016/j.chemgeo.2017.11.008. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

