Analysis of Signal Transduction Pathways Downstream M2 Receptor Activation: Effects on Schwann Cell Migration and Morphology
- PMID: 35207498
- PMCID: PMC8875146
- DOI: 10.3390/life12020211
Analysis of Signal Transduction Pathways Downstream M2 Receptor Activation: Effects on Schwann Cell Migration and Morphology
Abstract
Background: Schwann cells (SCs) express cholinergic receptors, suggesting a role of cholinergic signaling in the control of SC proliferation, differentiation and/or myelination. Our previous studies largely demonstrated that the pharmacological activation of the M2 muscarinic receptor subtype caused an inhibition of cell proliferation and promoted the expression of pro-myelinating differentiation genes. In order to elucidate the molecular signaling activated downstream the M2 receptor activation, in the present study we investigated the signal transduction pathways activated by the M2 orthosteric agonist arecaidine propargyl ester (APE) in SCs.
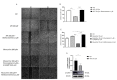
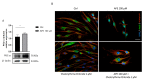
Methods: Using Western blot we analyzed some components of the noncanonical pathways involving β1-arrestin and PI3K/AKT/mTORC1 signaling. A wound healing assay was used to evaluate SC migration.
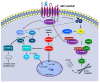
Results: Our results demonstrated that M2 receptor activation negatively modulated the PI3K/Akt/mTORC1 axis, possibly through β1-arrestin downregulation. The involvement of the mTORC1 complex was also supported by the decreased expression of its specific target p-p70 S6KThr389. Then, we also analyzed the expression of p-AMPKαthr172, a negative regulator of myelination that resulted in reduced levels after M2 agonist treatment. The analysis of cell migration and morphology allowed us to demonstrate that M2 receptor activation caused an arrest of SC migration and modified cell morphology probably by the modulation of β1-arrestin/cofilin-1 and PKCα expression, respectively.
Conclusions: The data obtained demonstrated that M2 receptor activation in addition to the canonical Gi protein-coupled pathway modulates noncanonical pathways involving the mTORC1 complex and other kinases whose activation may contribute to the inhibition of SC proliferation and migration and address SC differentiation.
Keywords: AMPK; M2 muscarinic receptor; Schwann cells; cell migration; mTOR pathway; β1-arrestin.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

Similar articles
-
α7 Nicotinic Acetylcholine Receptors May Improve Schwann Cell Regenerating Potential via Metabotropic Signaling Pathways.Cells. 2023 May 28;12(11):1494. doi: 10.3390/cells12111494. Cells. 2023. PMID: 37296615 Free PMC article.
-
M2 receptors activation modulates cell growth, migration and differentiation of rat Schwann-like adipose-derived stem cells.Cell Death Discov. 2019 May 3;5:92. doi: 10.1038/s41420-019-0174-6. eCollection 2019. Cell Death Discov. 2019. PMID: 31069117 Free PMC article.
-
Notch Signal Mediates the Cross-Interaction between M2 Muscarinic Acetylcholine Receptor and Neuregulin/ErbB Pathway: Effects on Schwann Cell Proliferation.Biomolecules. 2022 Feb 1;12(2):239. doi: 10.3390/biom12020239. Biomolecules. 2022. PMID: 35204740 Free PMC article.
-
Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship.Adv Biol Regul. 2019 May;72:51-62. doi: 10.1016/j.jbior.2019.03.003. Epub 2019 Apr 11. Adv Biol Regul. 2019. PMID: 31010692 Review.
-
Myelination and mTOR.Glia. 2018 Apr;66(4):693-707. doi: 10.1002/glia.23273. Epub 2017 Dec 6. Glia. 2018. PMID: 29210103 Free PMC article. Review.
Cited by
-
Emerging Roles of Cholinergic Receptors in Schwann Cell Development and Plasticity.Biomedicines. 2022 Dec 24;11(1):41. doi: 10.3390/biomedicines11010041. Biomedicines. 2022. PMID: 36672549 Free PMC article. Review.
-
β-Arrestin 2 as a Prognostic Indicator and Immunomodulatory Factor in Multiple Myeloma.Cells. 2025 Mar 26;14(7):496. doi: 10.3390/cells14070496. Cells. 2025. PMID: 40214450 Free PMC article.
-
Acetylcholine muscarinic M2 receptor maintains human Schwann-like adipose-derived phenotype in the absence of differentiating medium.Cell Death Discov. 2025 Apr 13;11(1):170. doi: 10.1038/s41420-025-02404-0. Cell Death Discov. 2025. PMID: 40222967 Free PMC article.
-
Muscarinic Cholinoreceptors in Skeletal Muscle: Localization and Functional Role.Acta Naturae. 2023 Oct-Dec;15(4):44-55. doi: 10.32607/actanaturae.25259. Acta Naturae. 2023. PMID: 38234599 Free PMC article.
-
Special Issue "G Protein-Coupled Receptors: Molecular Mechanisms Involved in Receptor Activation and Selectivity".Life (Basel). 2023 Jan 6;13(1):166. doi: 10.3390/life13010166. Life (Basel). 2023. PMID: 36676115 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

