Quantitative Fluorescence Imaging of Perfusion-An Algorithm to Predict Anastomotic Leakage
- PMID: 35207536
- PMCID: PMC8875734
- DOI: 10.3390/life12020249
Quantitative Fluorescence Imaging of Perfusion-An Algorithm to Predict Anastomotic Leakage
Abstract
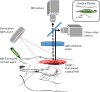
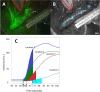
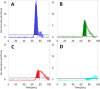
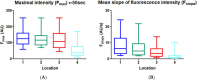
This study tests fluorescence imaging-derived quantitative parameters for perfusion evaluation of the gastric tube during surgery and correlates these parameters with patient outcomes in terms of anastomotic leakage. Poor fundus perfusion is seen as a major factor for the development of anastomotic leakage and strictures. Fluorescence perfusion imaging may reduce the incidence of complications. Parameters for the quantification of the fluorescence signal are still lacking. Quantitative parameters in terms of maximal intensity, mean slope and influx timepoint were tested for significant differences between four perfusion areas of the gastric tube in 22 patients with a repeated ANOVA test. These parameters were compared with patient outcomes. Maximal intensity, mean slope and influx timepoint were significantly different between the base of the gastric tube and the fundus (p < 0.0001). Patients who developed anastomotic leakage showed a mean slope of almost 0 in Location 4. The distance of the demarcation of ICG to the fundus was significantly higher in the three patients who developed anastomotic leakage (p < 0.0001). This study presents quantitative intra-operative perfusion imaging with fluorescence. Quantification of the fluorescence signal allows for early risk stratification of necrosis.
Keywords: anastomosis; fluorescence imaging; parameters; perfusion; surgery.
Conflict of interest statement
M.I.v.B.H. is consultant for Mylan, Johnson & Johnson, Alesi Surgical, BBraun and Medtronic, and received unrestricted research grants from Stryker. All fees paid to institution.
Figures



References
-
- Futier E., Robin E., Jabaudon M., Guerin R., Petit A., Bazin J.E., Constantin J.M., Vallet B. Central venous O(2) saturation and venous-to-arterial CO(2) difference as complementary tools for goal-directed therapy during high-risk surgery. Crit. Care. 2010;14:R193. doi: 10.1186/cc9310. - DOI - PMC - PubMed
-
- van Hagen P., Hulshof M.C., van Lanschot J.J., Steyerberg E.W., van Berge Henegouwen M.I., Wijnhoven B.P., Richel D.J., Nieuwenhuijzen G.A., Hospers G.A., Bonenkamp J.J., et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. - DOI - PubMed
-
- Rino Y., Yukawa N., Sato T., Yamamoto N., Tamagawa H., Hasegawa S., Oshima T., Yoshikawa T., Masuda M., Imada T. Visualization of blood supply route to the reconstructed stomach by indocyanine green fluorescence imaging during esophagectomy. BMC Med. Imaging. 2014;14:18. doi: 10.1186/1471-2342-14-18. - DOI - PMC - PubMed
-
- Shimada Y., Okumura T., Nagata T., Sawada S., Matsui K., Hori R., Yoshioka I., Yoshida T., Osada R., Tsukada K. Usefulness of blood supply visualization by indocyanine green fluorescence for reconstruction during esophagectomy. Esophagus. 2011;8:259–266. doi: 10.1007/s10388-011-0291-7. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

