Preliminary Acceptability of a Home-Based Peripheral Blood Collection Device for Viral Load Testing in the Context of Analytical Treatment Interruptions in HIV Cure Trials: Results from a Nationwide Survey in the United States
- PMID: 35207719
- PMCID: PMC8879991
- DOI: 10.3390/jpm12020231
Preliminary Acceptability of a Home-Based Peripheral Blood Collection Device for Viral Load Testing in the Context of Analytical Treatment Interruptions in HIV Cure Trials: Results from a Nationwide Survey in the United States
Abstract
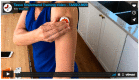
Frequent viral load testing is necessary during analytical treatment interruptions (ATIs) in HIV cure-directed clinical trials, though such may be burdensome and inconvenient to trial participants. We implemented a national, cross-sectional survey in the United States to examine the acceptability of a novel home-based peripheral blood collection device for HIV viral load testing. Between June and August 2021, we distributed an online survey to people with HIV (PWH) and community members, biomedical HIV cure researchers and HIV care providers. We performed descriptive analyses to summarize the results. We received 73 survey responses, with 51 from community members, 12 from biomedical HIV cure researchers and 10 from HIV care providers. Of those, 51 (70%) were cisgender men and 50 (68%) reported living with HIV. Most (>80% overall) indicated that the device would be helpful during ATI trials and they would feel comfortable using it themselves or recommending it to their patients/participants. Of the 50 PWH, 42 (84%) indicated they would use the device if they were participating in an ATI trial and 27 (54%) also expressed a willingness to use the device outside of HIV cure studies. Increasing sensitivity of viral load tests and pluri-potency of the device (CD4 count, chemistries) would augment acceptability. Survey findings provide evidence that viral load home testing would be an important adjunct to ongoing HIV cure-directed trials involving ATIs. Survey findings may help inform successful implementation and uptake of the device in the context of personalized HIV care.
Keywords: HIV cure research; acceptability; analytical treatment interruptions; home-based viral load; people with HIV; personalized medicine.
Conflict of interest statement
D.R., B.E., M.A., D.J.H., L.S., K.B. and B.J.H. and employed by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Figures








References
-
- Julg B., Dee L., Ananworanich J., Barouch D.H., Bar K., Caskey M., Colby D.J., Dawson L., Dong K.L., Dubé K., et al. Recommendations for Analytical Antiretroviral Treatment Interruptions in HIV Research Trials—Report of a Consensus Meeting. Lancet HIV. 2019;6:e259–e268. doi: 10.1016/S2352-3018(19)30052-9. - DOI - PMC - PubMed
-
- TAG Research Toward a Cure Trials. 2021. [(accessed on 29 November 2021)]. Available online: http://www.treatmentactiongroup.org/cure/trials.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

