Analysis of the Efficacy of Universal Screening of Coronavirus Disease with Antigen-Detecting Rapid Diagnostic Tests at Point-or-Care Settings and Sharing the Experience of Admission Protocol-A Pilot Study
- PMID: 35207807
- PMCID: PMC8876277
- DOI: 10.3390/jpm12020319
Analysis of the Efficacy of Universal Screening of Coronavirus Disease with Antigen-Detecting Rapid Diagnostic Tests at Point-or-Care Settings and Sharing the Experience of Admission Protocol-A Pilot Study
Abstract
Aims: To introduce the admission protocol of a COVID-19 specialized hospital outlined by the government, including the assessment of reverse transcription polymerase chain reaction (RT-PCR), low dose chest computed tomography (CT) and antigen-detecting rapid diagnostic test (Ag-RDT) for patient screening.
Materials and methods: This was a retrospective cohort study of 646 patients who were admitted between December 2020, and February 2021, during the third wave of COVID-19 in Korea. Ag-RDT and RT-PCR were routinely performed on all patients who required admission, and low-dose chest CT was performed on high-risk patients with associated symptoms. Any patients with high-risk COVID-19 infection according to the Ag-RDT test were quarantined alone in a negative pressured room, and those with low-risk COVID-19 infection remained in the preemptive quarantine room with or without negative pressure. The diagnostic values of the Ag-RDT test and associated cycle threshold (Ct) values of the RT-PCR test were subsequently evaluated.
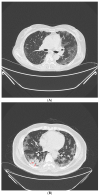
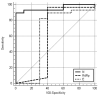
Results: In terms of the diagnostic value, the Ag-RDT for COVID-19 had a sensitivity of 68.3%, specificity of 99.5%, positive predictive value (PPV) of 90.3%, and negative predictive value (NPV) of 97.9%. For the 355 symptomatic patients with low-dose chest CT, the diagnostic values of combined evaluations had a sensitivity of 90.2%, specificity of 99.0%, PPV of 86.1%, and NPV of 99.3%. The cut-off Ct value for positive Ag-RDT was ≤25.67 for the N gene (sensitivity: 89.3%, specificity: 100%), which was regarded as a high viable virus in cell culture. There were no patients or medical staff who had COVID-19 in the hospital.
Conclusion: Appropriate patient care was possible by definitive triage of the area, according to the symptoms and using diagnostic tests. Screening protocols, including the Ag-RDT test and low-dose chest CT, could be helpful in emergency point-of-care settings.
Keywords: COVID-19; SARS-CoV-2; cross infection; infection control.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
Antigenic rapid test for SARS-CoV2 screening of individuals newly admitted to detention facilities: sensibility in an asymptomatic cohort.J Clin Virol Plus. 2021 Jun;1(1):100019. doi: 10.1016/j.jcvp.2021.100019. Epub 2021 May 11. J Clin Virol Plus. 2021. PMID: 35262006 Free PMC article.
-
Evaluation of the Abbott Panbio™ COVID-19 antigen detection rapid diagnostic test among healthcare workers in elderly care.PLoS One. 2023 Feb 24;18(2):e0276244. doi: 10.1371/journal.pone.0276244. eCollection 2023. PLoS One. 2023. PMID: 36827362 Free PMC article.
-
Rapid Antigen Test Combined with Chest Computed Tomography to Rule Out COVID-19 in Patients Admitted to the Emergency Department.J Clin Med. 2021 Aug 4;10(16):3455. doi: 10.3390/jcm10163455. J Clin Med. 2021. PMID: 34441750 Free PMC article.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. PMID: 32997361 Updated.
-
Diagnostic Performance of Antigen Rapid Diagnostic Tests, Chest Computed Tomography, and Lung Point-of-Care-Ultrasonography for SARS-CoV-2 Compared with RT-PCR Testing: A Systematic Review and Network Meta-Analysis.Diagnostics (Basel). 2022 May 24;12(6):1302. doi: 10.3390/diagnostics12061302. Diagnostics (Basel). 2022. PMID: 35741112 Free PMC article. Review.
Cited by
-
Comparison of diagnostic accuracy of rapid antigen tests for COVID-19 compared to the viral genetic test in adults: a systematic review and meta-analysis.JBI Evid Synth. 2024 Oct 1;22(10):1939-2002. doi: 10.11124/JBIES-23-00291. JBI Evid Synth. 2024. PMID: 39188132 Free PMC article.
References
-
- Kim J.Y., Choe P.G., Oh Y., Oh K.J., Kim J., Park S.J., Park J.H., Na H.K., Oh M.-D. The First Case of 2019 Novel Coronavirus Pneumonia Imported into Korea from Wuhan, China: Implication for Infection Prevention and Control Measures. J. Korean Med. Sci. 2020;35:e61. doi: 10.3346/jkms.2020.35.e61. - DOI - PMC - PubMed
-
- Ji W., Huh K., Ko K.-P., Im J.-S., Jung J., Kang M., Hong J., Bae G.H., Lee R., Na Y., et al. Effect of Underlying Comorbidities on the Infection and Severity of COVID-19 in Korea: A Nationwide Case-Control Study. J. Korean Med. Sci. 2020;35:e237. doi: 10.3346/jkms.2020.35.e237. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

