Homologous Recombination Deficiency (HRD) and BRCA 1/2 Gene Mutation for Predicting the Effect of Platinum-Based Neoadjuvant Chemotherapy of Early-Stage Triple-Negative Breast Cancer (TNBC): A Systematic Review and Meta-Analysis
- PMID: 35207810
- PMCID: PMC8876589
- DOI: 10.3390/jpm12020323
Homologous Recombination Deficiency (HRD) and BRCA 1/2 Gene Mutation for Predicting the Effect of Platinum-Based Neoadjuvant Chemotherapy of Early-Stage Triple-Negative Breast Cancer (TNBC): A Systematic Review and Meta-Analysis
Abstract
Background: Platinum-based agents may benefit patients with triple-negative breast cancer (TNBC) whose tumors are dysfunctional in DNA repair mechanisms associated with the homologous recombination repair (HRR) genes. The purpose of this meta-analysis was to assess the values of BRCA1/2 and homologous recombination deficiency (HRD) in the prediction of the pathological complete response (pCR) rates of patients with TNBC treated with platinum-based neoadjuvant chemotherapy (NAC).
Patients and methods: Patients with TNBC with BRCA or HRD status from platinum-based NAC trials were analyzed. The odds ratios (ORs) with 95% confidence intervals (CI) for the identified studies were calculated.
Results: 13 eligible studies between January 2000 and September 2021 were included through systematic literature searches of Embase, PubMed, Cochrane, and Web of Science databases. In 12 trials with BRCA status, 629 of 1266 (49.7%) patients with TNBC achieved pCR with platinum-based NAC, including 134 out of 222 (60.4%) BRCA1/2-mutated patients and 495 out of 1044 (47.4%) BRCA wildtype patients (OR, 1.62; 95% CI, 1.20-2.20). The prevalence of HRD was higher than BRCA1/2 mutations in patients with TNBC (69.2% vs. 17.5%). In six trials with HRD information, pCR rates of HRD-positive patients with TNBC were significantly higher than those of HRD-negative patients with TNBC (241/412, 58.5% vs. 60/183, 32.8%, OR, 3.01; 95% CI, 2.07-4.39, p < 0.001).
Conclusions: BRCA1/2-mutated and HRD-positive patients with TNBC could benefit from platinum-based NAC. In the future, a prospective study using unified HRD testing criteria is warranted for further investigation.
Keywords: BRCA; homologous recombination deficiency; neoadjuvant chemotherapy; platinum agents; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
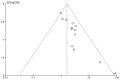
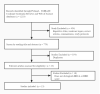

Similar articles
-
Homologous recombination deficiency predicts the response to platinum-based neoadjuvant chemotherapy in early-stage triple-negative breast cancer patients: a systematic review and meta-analysis.Ther Adv Med Oncol. 2022 May 7;14:17588359221096253. doi: 10.1177/17588359221096253. eCollection 2022. Ther Adv Med Oncol. 2022. PMID: 35547093 Free PMC article.
-
Homologous recombination deficiency (HRD) status predicts response to standard neoadjuvant chemotherapy in patients with triple-negative or BRCA1/2 mutation-associated breast cancer.Breast Cancer Res Treat. 2018 Apr;168(3):625-630. doi: 10.1007/s10549-017-4624-7. Epub 2017 Dec 23. Breast Cancer Res Treat. 2018. PMID: 29275435
-
Association of Tumor-Infiltrating Lymphocytes with Homologous Recombination Deficiency and BRCA1/2 Status in Patients with Early Triple-Negative Breast Cancer: A Pooled Analysis.Clin Cancer Res. 2020 Jun 1;26(11):2704-2710. doi: 10.1158/1078-0432.CCR-19-0664. Epub 2019 Dec 3. Clin Cancer Res. 2020. PMID: 31796517
-
The effect of neoadjuvant platinum-based chemotherapy in BRCA mutated triple negative breast cancers -systematic review and meta-analysis.Hered Cancer Clin Pract. 2019 Mar 25;17:11. doi: 10.1186/s13053-019-0111-y. eCollection 2019. Hered Cancer Clin Pract. 2019. PMID: 30962858 Free PMC article. Review.
-
Predictive biomarkers for triple negative breast cancer treated with platinum-based chemotherapy.Cancer Biol Ther. 2017 Jun 3;18(6):369-378. doi: 10.1080/15384047.2017.1323582. Epub 2017 May 11. Cancer Biol Ther. 2017. PMID: 28494179 Free PMC article. Review.
Cited by
-
Clinical Utility of Genomic Tests Evaluating Homologous Recombination Repair Deficiency (HRD) for Treatment Decisions in Early and Metastatic Breast Cancer.Cancers (Basel). 2023 Feb 18;15(4):1299. doi: 10.3390/cancers15041299. Cancers (Basel). 2023. PMID: 36831640 Free PMC article. Review.
-
Real-world data in patients with BRCA mutated breast cancer treated with poly (ADP-ribose) polymerase inhibitors.Ecancermedicalscience. 2023 Nov 21;17:1633. doi: 10.3332/ecancer.2023.1633. eCollection 2023. Ecancermedicalscience. 2023. PMID: 38414963 Free PMC article. Review.
-
Association between homologous recombination deficiency and outcomes with platinum and platinum-free chemotherapy in patients with triple-negative breast cancer.Cancer Biol Med. 2023 Mar 2;20(2):155-68. doi: 10.20892/j.issn.2095-3941.2022.0525. Cancer Biol Med. 2023. PMID: 36861447 Free PMC article.
-
Bibliometric analysis of phosphoglycerate kinase 1 expression in breast cancer and its distinct upregulation in triple-negative breast cancer.World J Clin Oncol. 2024 Jul 24;15(7):867-894. doi: 10.5306/wjco.v15.i7.867. World J Clin Oncol. 2024. PMID: 39071464 Free PMC article.
-
Clinical Utility of Whole-Genome Analysis as One-for-All Test for Breast Cancer: A Case Series.Case Rep Oncol. 2024 Feb 23;17(1):317-328. doi: 10.1159/000536087. eCollection 2024 Jan-Dec. Case Rep Oncol. 2024. PMID: 38404405 Free PMC article.
References
-
- Liedtke C., Mazouni C., Hess K.R., André F., Tordai A., Mejia J.A., Symmans W.F., Gonzalez-Angulo A.M., Hennessy B., Green M., et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. - DOI - PubMed
-
- Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N., Bonnefoi H., Cameron D., Gianni L., Valagussa P., et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. - DOI - PubMed
-
- Kalra M., Tong Y., Jones D.R., Walsh T., Danso M.A., Ma C.X., Silverman P., King M.C., Badve S.S., Perkins S.M., et al. Cisplatin +/− rucaparib after preoperative chemotherapy in patients with triple-negative or BRCA mutated breast cancer. NPJ Breast Cancer. 2021;7:29. doi: 10.1038/s41523-021-00240-w. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

